Degenerative Myelopathy (DM) and
the Cavalier King Charles Spaniel
-
What It Is

- Symptoms
- Diagnosis
- DNA Testing
- Treatment
- Breeders' Responsibilities
- Research News
- Related Links
- Veterinary Resources
Canine degenerative myelopathy (CDM or DM)* is a crippling and ultimately fatal neurological disease of the spinal cord, the symptoms of which begin to occur around age 8 years or older in most patients. "Myelopathy" refers to a disease of the spinal cord. "Degenerative" means progressive.
*CDM is also known as chronic degenerative radiculomyelopathy.
What It Is
Myelopathy is the general name for any injury to the spinal cord, including its degeneration, a spinal disc disorder, inflammation, or a tumor. Degenerative myelopathy is a compression of the spinal cord, which in the cavalier King Charles spaniel is caused by a mutation of the SOD1 (superoxide dismutase 1) gene. CDM is a chronic and progressive disorder which cannot be cured and results in lameness and eventual paralysis of the hind legs, followed by further muscle control deterioration. The disease affects the T3 to L3 segments of the spinal cord. It is considered to be the canine equivalent to amyotrophic lateral sclerosis (ALS -- Lou Gehrig's disease) in humans.
Prior to a
2014 study, the cavalier King Charles spaniel was not believed to
have a high prevalance rate for
 CDM. According to pevalence
rates which were calculated on the number of dogs presented to veterinary
teaching hospitals in North America between January 1990 and December
1999, CDM is most common in German
shepherd dogs (prevalence rate 2.01%) and also common in Pembroke Welsh Corgis
(prevalence rate 0.58%), boxers (prevalence rate 0.59%),
Chesapeake Bay retrievers (prevalence rate 0.83%), Rhodesian ridgebacks
(prevalence rate 0.74%) and collie (prevalence rate 0.38%), and less common in
several other breeds, including the cavalier.
CDM. According to pevalence
rates which were calculated on the number of dogs presented to veterinary
teaching hospitals in North America between January 1990 and December
1999, CDM is most common in German
shepherd dogs (prevalence rate 2.01%) and also common in Pembroke Welsh Corgis
(prevalence rate 0.58%), boxers (prevalence rate 0.59%),
Chesapeake Bay retrievers (prevalence rate 0.83%), Rhodesian ridgebacks
(prevalence rate 0.74%) and collie (prevalence rate 0.38%), and less common in
several other breeds, including the cavalier.
However, in that 2014 study, a mutation of the SOD1 (superoxide dismutase 1) gene has been identified in the cavalier breed. The exact frequency of this disease and approximate age of disease onset are unreported for the CKCS, but in that study, 37% out of 73 cavaliers tested were carriers of the mutation, and 49.3% were at-risk or affected. In that study, however, there is no indication as to how the 73 cavaliers were selected. These extremely high percentages are vastly different from objective breed-wide statistical studies, such as the UK study from 1990 to 1999, which showed the highest percentage of affected German shepherd dogs at 2.01%.
The SOD1 mutation appears to cause a mis-folding of proteins, resulting in an accumulation of toxic by-products in the axon (nerve fibers which transmit information to different neurons). These toxic by-products disrupt axoplasmic flow, eventually destroying myelin and ultimately replacing normal axons with astrogliosis (an abnormal increase in the number of astrocytes due to the destruction of nearby neurons).
Interestingly, it appears that not all cavaliers carrying two copies of the SOD1 mutation will develop the disease. So, there is much more to be learned about this disorder in the CKCS.
CDM initially attacks the nerve cells (neurons) in the spinal cord which control muscle movements in the hindquarters, causing at least partial loss of coordination and voluntary movement and sensory function (ataxia).
More detailed information is available on Dr. Clare Rusbridge's website. See, also, the Cure4DM website.
RETURN TO TOP
Symptoms
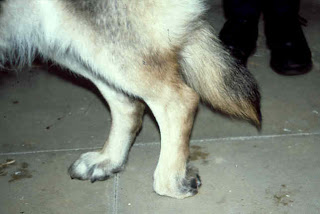 The symptoms of CDM are chronic and insidiously progressive and thus
far cannot be cured or successfully managed long-term. CDM often begins with
a painless lack of voluntary coordination
of muscle movements and sensation in the pelvic region. A wobbly gait
and scuffing of one
or both hind paws are common signs at the onset of the disease,
proceeding to worn, bleeding claws. (See photo at right.) Staggering and asymmetric
spastic movements are typical.
The symptoms of CDM are chronic and insidiously progressive and thus
far cannot be cured or successfully managed long-term. CDM often begins with
a painless lack of voluntary coordination
of muscle movements and sensation in the pelvic region. A wobbly gait
and scuffing of one
or both hind paws are common signs at the onset of the disease,
proceeding to worn, bleeding claws. (See photo at right.) Staggering and asymmetric
spastic movements are typical.
 The disease itself does not produce pain, but the physical injuries it
can cause to the dog certainly can be painful. See the YouTube video
"Charlie's Degenerative Myelopathy Progression".
The disease itself does not produce pain, but the physical injuries it
can cause to the dog certainly can be painful. See the YouTube video
"Charlie's Degenerative Myelopathy Progression".
The disorder may be expected to progress to partial paralysis of the rear legs within 6 to 9 months from the onset of symptoms. Usually within 14 to 24 months from the onset, the loss of muscle and sensory control extends to the forelimbs. Loss of muscle mass follows, and the disorder advances to inhibit swallowing and the ability to bark, followed by urinary and fecal continence. If the affected dog is not euthanized, respiratory difficulty may be expected to lead to respiratory failure.
RETURN TO TOP
Diagnosis
 Diagnosis of CDM can be very difficult because it calls for the
elimination of other mimicking disorders. Among them is
intervertebral disc disease, which is common in
the cavalier King Charles spaniel, and
arthritis because of the dog's
usual advanced age, as well as degenerative lumbosacral stenosis, spinal
cord neoplasia, spinal cysts, boney compressive lesions, caudal cervical
spinal cord, orthopedic problems, and
infections. Routine blood work, x-rays, and biochemistry typically will
reveal no abnormalities. Cerebrospinal fluid (CSF) analysis is limited
to exclude other neurological disorders.
Diagnosis of CDM can be very difficult because it calls for the
elimination of other mimicking disorders. Among them is
intervertebral disc disease, which is common in
the cavalier King Charles spaniel, and
arthritis because of the dog's
usual advanced age, as well as degenerative lumbosacral stenosis, spinal
cord neoplasia, spinal cysts, boney compressive lesions, caudal cervical
spinal cord, orthopedic problems, and
infections. Routine blood work, x-rays, and biochemistry typically will
reveal no abnormalities. Cerebrospinal fluid (CSF) analysis is limited
to exclude other neurological disorders.
In an August 2023 article, a survey of 190 veterinary neurologists found that most of them diagnose by DNA testing for the mutation of the SOD1 gene (see details below) and by either Magnetic resonance imaging (MRI)or computerised tomography (CT) scans, but MRI and CT are performed only to rule out other diseases, particularly intervertebral disc disease (IVDD).
Electro-physiological studies such as electromyography (EMG) and motor nerve conduction velocities (MNCV) do not detect abnormalities early in the disease, but EMG may show multifocal spontaneous activity in later stages.
The only way to confirm the diagnosis of CDM is by examination of the spinal cord after the dog dies.
In a February 2017 article, an American research team studied the fluid biomarker phosphorylated neurofilament heavy (pNF-H), a structural protein of myelinated axons, of 53 DM-affected dogs (no CKCSs) in various stages of DM. Their results suggest that pNF-H is a sensitive biomarker for diagnosis of DM, but that further study with a larger cohort is recommended.
RETURN TO TOP
DNA Testing
 CDM is considered to be genetically caused, based upon the recurrance
of the disease in family generations. A
2009 study has revealed a point mutation in exon two of the
canine SOD1 (superoxide dismutase 1) gene which is consistent
with the disorder. Homozygosity of the SOD1 mutation is
strongly associated with CDM. However, this mutation is also found in
asymptomatic dogs. In other words, unlike many other genetic mutations
in dogs, such as episodic falling
syndrome and curly coat syndrome in
cavaliers, confirmation that a dog has two copies of the SOD1
mutation is not confirmation that the dog has CDM. Other possible
contributing or alternative causes of neurological disease could be
causing some of the same symptoms.
CDM is considered to be genetically caused, based upon the recurrance
of the disease in family generations. A
2009 study has revealed a point mutation in exon two of the
canine SOD1 (superoxide dismutase 1) gene which is consistent
with the disorder. Homozygosity of the SOD1 mutation is
strongly associated with CDM. However, this mutation is also found in
asymptomatic dogs. In other words, unlike many other genetic mutations
in dogs, such as episodic falling
syndrome and curly coat syndrome in
cavaliers, confirmation that a dog has two copies of the SOD1
mutation is not confirmation that the dog has CDM. Other possible
contributing or alternative causes of neurological disease could be
causing some of the same symptoms.
Nevertheless, genotype analysis based on this mutation has been
developed and is commercially available as a diagnostic test for CDM.
The SOD1 genotyping test, in conjunction with clinical
investigations, has significantly
improved the disease's prediction.
The test will enable breeders to identify dogs that carry the genetic
risk factor so that they can avoid matings that will produce puppies
that are at increased risk of developing CDM as they age.
In a 2014 study, the mutation of the SOD1 gene has been identified in the cavalier King Charles spaniel. The exact frequency of this disease and approximate age of disease onset are unreported for the CKCS. However, in that study, 37% out of 73 cavaliers tested were carriers of the mutation, and 49.3% were at-risk or affected. In that study, however, there is no indication as to how the 73 cavaliers were selected. These extremely high percentages are vastly different from objective breed-wide statistical studies, such as the UK study from 1990 to 1999, which showed the highest percentage of affected German shepherd dogs at 2.01%.
The genetic test of the SOD1 gene in cavaliers should determine whether a dog is a genetic carrier of CDM. The disorder is believed to be inherited in an autosomal recessive manner in dogs, meaning that they must receive two copies of the mutated gene -- one from each parent -- to develop the disease. In general, carrier dogs do not have features of the disease but when bred with another carrier of the same mutation, there is a risk of producing affected offspring. Theoretically, each puppy that is born to this pairing has a 25% chance of inheriting two copies of the SOD1 gene mutation and being at risk for the disease, and a 50% chance of inheriting one copy and being a carrier of the SOD1 gene mutation.
Genetic testing laboratories which test for this mutation causing CDM include:
• American Kennel Club (AKC)
• Canine Genetic Diseases Network, University of Missouri
• Canine Genetic Testing (CAGT), University of Cambridge Dept. of Veterinary Medicine
• DDC DNA Diagnostics Center
• Dog Breeding Science (Australia)
• Embark, Cornell University College of Veterinary Medicine
• GenSol Animal Diagnostics
• Laboklin
• MyDogDNA, Wisdom Panel
• Orivet
• Paw Print Genetics
• Veterinary Genetics Laboratory, University of California - Davis Veterinary Medicine
![]() Watch
this YouTube video to learn how to properly use a swab to obtain DNA from
your dog. See our Genetic Testing webpage for
more information about DNA testing.
Watch
this YouTube video to learn how to properly use a swab to obtain DNA from
your dog. See our Genetic Testing webpage for
more information about DNA testing.
RETURN TO TOP
Treatment
 CDM
cannot be cured. There are no proven effective therapies to halt or slow
its progression. An aminocapoic acid (EACA) and
n-acetylcysteine (NAC),
combined with vitamins C and E, may slow
the progression, but these treatments are experimental and
controversial.
CDM
cannot be cured. There are no proven effective therapies to halt or slow
its progression. An aminocapoic acid (EACA) and
n-acetylcysteine (NAC),
combined with vitamins C and E, may slow
the progression, but these treatments are experimental and
controversial.
Nevertheless, it is possible to maintain the dog's quality of life for a short time through nutrition and exercise. Well-balanced diets of fresh, unprocessed (no dry foods or kibble) food have been recommended. Regular exercise, such as walking and swimming, is recommended to maintain the dog's ability to walk.
Pain medications, such as gabapentin and non-steroidal anti-inflammatory drugs (NSAIDs), have been prescribed with some success. See this August 2020 article regarding the dosages of gabapentin.
Riluzole, a drug for treating Amyotrophic Lateral Sclerosis (ALS) in humans, is being tested on dogs to determine if it could delay the progression of DM and/or improve the dogs' quality of life. This drug inhibits glutamate release, reducing glutamate excitotoxicity, which is implicated in motor neuron degeneration in DM. In the studies, riluzole is injected into the spinal canal or given orally in capsules.
Curcumin, an active ingredient in turmeric, a spice used in curry, may serve to slow the progression of DM, but the evidence thus far is very weak. Curcumin which has been extracted from the turmeric plant is far more potent than turmeric powder itself. This spice is known to be poorly absorbed in the digestive tract, so processes have been developed to improve the bioavailability of curcumin. In a September 2021 article, Japanese researchers reported a retrospective study of 40 DM-affected Pembroke Welsh corgis, 8 of which were given curcumin supplements by their owners, and the remaining 32 dogs were not. They stated:
"We found that dogs with DM treated with curcumin had a significantly longer survival time than those who did not receive this treatment. Curcumin is a biologically potent polyphenolic compound that has various effects on the central nervous system, including neuroprotective effects such as antioxidant and anti-inflammatory activities."
Supplementing the dog's diet with L-carnitine and Omega 3 fatty acids (EPA, DHA) may be helpful.
Physical therapies, including active and passive exercise, massage, hydrotherapy, and paw protection, are designed to maintain joint range of motion, may slow development of muscle atrophy, maintain neuromuscular function, and improve quality of life and may prolong the length of time that the dog remains mobile and increase survival time. See this July 2006 article and this August 2023 article.
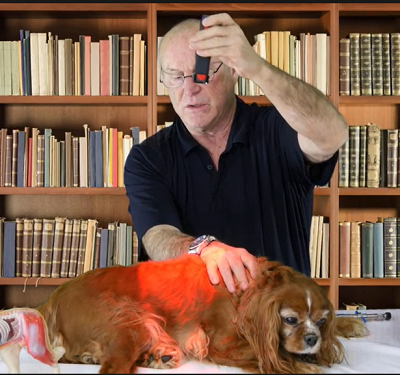
Acupuncture, laser therapy (see photo below), and herbal therapies may help slow CDM's progression, improve the dog's quality of life, and relieve any gastro-intestinal symptoms. See this 2015 article for recommended alternative care treatments. See the YouTube video, "Degenerative Myelopathy Treatment Using the LZ30-z Laser". Herbs may include bupleurum and those containing milk thistle.
 Assistive walking devices such as lifting harnesses (e.g.,
Help'EmUp and
Eddies Wheels) and wheeled harnesses
(K9Carts) are
available to provide affected dogs with some mobility early in the
progression of CDM. See also, the
YouTube video "Daisy Gets Her Wheels".
Assistive walking devices such as lifting harnesses (e.g.,
Help'EmUp and
Eddies Wheels) and wheeled harnesses
(K9Carts) are
available to provide affected dogs with some mobility early in the
progression of CDM. See also, the
YouTube video "Daisy Gets Her Wheels".
In the disease's final stage, respiratory failure, supplemental oxygen may serve to ease the discomfort.
RETURN TO TOP
Breeders' Responsibilities
Canine degenerative myelopathy is considered a heritable disease, and therefore affected cavaliers should not be bred. All cavalier breeding stock should be tested for CDM. See the DNA Testing section above for details.
RETURN TO TOP
Research News
August 2020:
Colorado State Univ. researchers find gabapentin is
well-tolerated at high dosages for dogs in chronic pain.
 In
an
August 2020 article, a team of Colorado State University researchers
(Lily V Davis, Peter W Hellyer, Robin A Downing, Lori R Kogan [right])
studied the treatment records of 240 dogs suffering chronic pain due to
a variety of diagnosed causes, including osteoarthritis (84.6%),
generalized, nonspecific back pain (9.2%), intervertebral disk disease
(7.5%), degenerative myelopathy (4.6%), and 56.67% not having a
definitive anatomical cause for their pain. They
report finding that:
In
an
August 2020 article, a team of Colorado State University researchers
(Lily V Davis, Peter W Hellyer, Robin A Downing, Lori R Kogan [right])
studied the treatment records of 240 dogs suffering chronic pain due to
a variety of diagnosed causes, including osteoarthritis (84.6%),
generalized, nonspecific back pain (9.2%), intervertebral disk disease
(7.5%), degenerative myelopathy (4.6%), and 56.67% not having a
definitive anatomical cause for their pain. They
report finding that:
"The results from this case series suggest that gabapentin is well-tolerated at much higher doses than what is typically prescribed. Side effects were uncommon, with no clear pattern based on dose, or dog size or age. Therefore, like many other analgesic medications, the efficacy of gabapentin appears patient-specific and should be dosed to effect until side effects are noted or analgesia is achieved.
July 2017:
Neurologist Dr. Coates of Univ. of Missouri needs DM-affected
dogs for clinical trial.
 Dr.
Joan Coates (right), board certified veterinary neurologist
at the University of Missouri in Columbia, Missour, is seeking dogs
affected with degenerative myelopathy (DM) for a clinical trial to
evaluate a new drug intended to slow the progression of DM. The dogs
will be given an "antisense oligonucleotide" (ASO) that will repress the
production of the protein superoxide dismutase 1 (SOD1). She advises
that the drug has been tested in a preclinical setting for safety in
dogs. The drug will be injected into the spinal fluid at the low lumbar
region of the spinal cord. The study will be randomized and
double-blinded; neither the investigator nor the pet owner will know
whether the dog receives the drug. Eight dogs will receive the drug and
four dogs will receive a placebo. Based on the randomization design,
each dog has a 67 percent chance of receiving the therapy.
Details of the qualifications for the study are described at this link.
Contact Dr. Coates at 573-882-7821, or coatesj@missouri.edu
Dr.
Joan Coates (right), board certified veterinary neurologist
at the University of Missouri in Columbia, Missour, is seeking dogs
affected with degenerative myelopathy (DM) for a clinical trial to
evaluate a new drug intended to slow the progression of DM. The dogs
will be given an "antisense oligonucleotide" (ASO) that will repress the
production of the protein superoxide dismutase 1 (SOD1). She advises
that the drug has been tested in a preclinical setting for safety in
dogs. The drug will be injected into the spinal fluid at the low lumbar
region of the spinal cord. The study will be randomized and
double-blinded; neither the investigator nor the pet owner will know
whether the dog receives the drug. Eight dogs will receive the drug and
four dogs will receive a placebo. Based on the randomization design,
each dog has a 67 percent chance of receiving the therapy.
Details of the qualifications for the study are described at this link.
Contact Dr. Coates at 573-882-7821, or coatesj@missouri.edu
March 2017:
Cummings School (Tufts) researchers need DM-affected dogs for an
engineered adenovirus study. Dr. Dominik Faissler
(right), a veterinary neurologist at Cummings School at Tufts
University in Massachusetts, is leading a current study of degenerative
myelopathy, and he needs dogs to volunteer for an experimental remedy.
The dogs in the trial receive a single spinal fluid injection of an
engineered adenovirus--from a family of viruses that can infect the
nervous system. The engineered virus was designed to breach the
blood-brain barrier to deliver DNA particles that turn off--or
silence--the mutated SOD1 gene. Dogs in the trial, which began in
December 2016, are checked every three months and undergo tests, which
are videotaped, to assess their neurological and motor function.
Veterinarians and pet owners interested in participating in the study
should email neuro@tufts.edu or
call Kellye St. John at 508-887-4839.
Dr. Dominik Faissler
(right), a veterinary neurologist at Cummings School at Tufts
University in Massachusetts, is leading a current study of degenerative
myelopathy, and he needs dogs to volunteer for an experimental remedy.
The dogs in the trial receive a single spinal fluid injection of an
engineered adenovirus--from a family of viruses that can infect the
nervous system. The engineered virus was designed to breach the
blood-brain barrier to deliver DNA particles that turn off--or
silence--the mutated SOD1 gene. Dogs in the trial, which began in
December 2016, are checked every three months and undergo tests, which
are videotaped, to assess their neurological and motor function.
Veterinarians and pet owners interested in participating in the study
should email neuro@tufts.edu or
call Kellye St. John at 508-887-4839.
February 2017:
 USA researchers find promising CSF biomaker for early diagnosis
of DM. In a
February 2017 article, an American research team (C.M. Toedebusch,
M.D. Bachrach, V.B. Garcia, G.C. Johnson, M.L. Katz, G. Shaw, J.R.
Coates, Michael L. Garcia [right]) studied the fluid
biomarker phosphorylated neurofilament heavy (pNF-H), a structural
protein of myelinated axons, of 53 DM-affected dogs (no CKCSs) in
various stages of DM. Their results suggest that pNF-H is a sensitive
biomarker for diagnosis of DM, but that further study with a larger
cohort is recommended.
USA researchers find promising CSF biomaker for early diagnosis
of DM. In a
February 2017 article, an American research team (C.M. Toedebusch,
M.D. Bachrach, V.B. Garcia, G.C. Johnson, M.L. Katz, G. Shaw, J.R.
Coates, Michael L. Garcia [right]) studied the fluid
biomarker phosphorylated neurofilament heavy (pNF-H), a structural
protein of myelinated axons, of 53 DM-affected dogs (no CKCSs) in
various stages of DM. Their results suggest that pNF-H is a sensitive
biomarker for diagnosis of DM, but that further study with a larger
cohort is recommended.
February 2014:
 Mutant gene
for canine degenerative myelopathy is identified in the CKCS and other
breeds. In a
February 2014 study, researchers (R. Zeng, J.R. Coates, G.C.
Johnson, L. Hansen, T. Awano, A. Kolicheski, E. Ivansson, M. Perloski,
K. Lindblad-Toh, D.P. O'Brien, J. Guo, M.L. Katz, Gary S. Johnson
[right]) have identified the mutation of the
Sod1 gene believed to be associated with canine degenerative myelopathy,
in the cavalier King Charles spaniel and 123 other purebred breeds. 73
cavaliers were included in the study, of which 49.3% were found to be
have a pair of the mutation and were therefore deemed to be "at risk"
for CDM, and another 37% of CKCSs were found to have one of the
mutations and therefore deemed to be "carriers".
Mutant gene
for canine degenerative myelopathy is identified in the CKCS and other
breeds. In a
February 2014 study, researchers (R. Zeng, J.R. Coates, G.C.
Johnson, L. Hansen, T. Awano, A. Kolicheski, E. Ivansson, M. Perloski,
K. Lindblad-Toh, D.P. O'Brien, J. Guo, M.L. Katz, Gary S. Johnson
[right]) have identified the mutation of the
Sod1 gene believed to be associated with canine degenerative myelopathy,
in the cavalier King Charles spaniel and 123 other purebred breeds. 73
cavaliers were included in the study, of which 49.3% were found to be
have a pair of the mutation and were therefore deemed to be "at risk"
for CDM, and another 37% of CKCSs were found to have one of the
mutations and therefore deemed to be "carriers".
RETURN TO TOP
RETURN TO TOP
Veterinary Resources
Degenerative myelopathy in the aging German Shepherd dog: clinical and pathologic findings. Averill D.R. Jr. J Am Vet Med Assoc. June 1973;62(12):1045-51.
Degenerative Myelopathy. Roger M. Clemmons. Small Anim. Pract. July 1992;22(4):965-971. Quote: Degenerative myelopathy (DM) is a neurodegenerative disease that is seen particularly in the German Shepherd. DM is a specific disorder characterized by widespread myelin and axon loss beginning in the thoracolumbar area of the spinal cord. The age of onset of DM is between 5 and 7 years. It may be confused with other disorders, including intervertebral disc protrusions, lumbosacral stenosis, and hip dysplasia, which also occur in older dogs. Most of the information about the pathophysiology and immunology of DM has come from studies of German Shepherds afflicted with the disease. ... DM in the German Shepherd is an immune-related disorder whose clinical signs are explained by a widespread degeneration of the white matter pathways in the thoracolumbar spinal cord. Therapy includes exercise, vitamin supplementation, and EACA [aminocaproic acid] medication. Avoiding unnecessary surgical procedures is also important to preclude permanent deterioration that can result following surgery in OM patients. In dogs other than German Shepherds, other identifiable causes should be treated. Additional confirmation of the diagnosis of DM may be assisted by performing cell-mediated immune studies or other serodiagnostic tests as they become available.
Central nervous system pathology in 25 dogs with chronic degenerative radiculomyelopathy. R E. J. Johnston, J. A. Barrie, M. C. Mcculloch, T. J. Anderson, I. R. Griffiths. Vet.Rec. 2000;146:629-633. Quote: "The neuropathology of 20 German shepherd dogs and five German shepherd dog crosses with chronic degenerative radiculomyelopathy were analysed by conventional techniques, immunocytochemistry and electron microscopy. There were previously unrecognised changes in brain nuclei. In the spinal cord, both motor and sensory tracts were involved, principally in their more distal regions. Wallerian degeneration affected the corticorubrospinal pathways in the lateral columns and the ventral funiculi, predominantly in the caudal thoracic and lumbar segments, although more cranial involvement was also observed. The dorsal columns were affected in the caudal lumbar region and the cervical fasciculus gracilis. The regional distribution was variable between cases. Within the brain, abnormalities, including chromatolysis, gliosis and neuronal loss were observed in the red nucleus, lateral vestibular nucleus and, occasionally, in the dentate nucleus. The changes in brain nuclei were compared with those found in dogs at various times after a focal spinal injury. The neuronal changes in the brain may be related to the primary site of damage, and possible aetiological mechanisms are discussed."
Daily Controlled Physiotherapy Increases Survival Time in Dogs with Suspected Degenerative Myelopathy. I. Kathmann, S. Cizinauskas, M.G. Doherr, F. Steffen, A. Jaggy. J.Vet.Int.Med. July 2006;20(4):927-932. Quote: "The purposes of the study reported here were to evaluate the signalment and clinical presentation in 50 dogs with degenerative myelopathy, to evaluate whether mean survival time was significantly affected by various means of physiotherapy performed in 22 dogs, and to determine whether neurologic status, anatomic localization, or age at onset had an influence on survival time in dogs that received physiotherapy. We found a significant (P < .05) breed predisposition for the German Shepherd Dog, Kuvasz, Hovawart, and Bernese Mountain Dog. Mean age at diagnosis was 9.1 years, and both sexes were affected equally. The anatomic localization of the lesion was spinal cord segment T3-L3 in 56% (n = 28) and L3-S3 in 44% (n = 22) of the dogs. Animals that received intensive (n = 9) physiotherapy had longer (P < .05) survival time (mean 255 days), compared with that for animals with moderate (n = 6; mean 130 days) or no (n = 7; mean 55 days) physiotherapy. In addition, our results indicate that affected dogs which received physiotherapy remained ambulatory longer than did animals that did not receive physical treatment."
Efficient mapping of mendelian traits in dogs through genome-wide association. Elinor K Karlsson, Izabella Baranowska, Claire M Wade1, Nicolette H C Salmon Hillbertz, Michael C Zody, Nathan Anderson, Tara M Biagi, Nick Patterson, Gerli Rosengren Pielberg, Edward J Kulbokas III, Kenine E Comstock, Evan T Keller, Jill P Mesirov, Henrik von Euler, Olle Kampe, Åke Hedhammar, Eric S Lander, Goran Andersson, Leif Andersson, Kerstin Lindblad-Toh. Nature Genetics. Sept. 2007;39:1321 - 1328. Quote: "With several hundred genetic diseases and an advantageous genome structure, dogs are ideal for mapping genes that cause disease. Here we report the development of a genotyping array with ~27,000 SNPs and show that genome-wide association mapping of mendelian traits in dog breeds can be achieved with only ~20 dogs. Specifically, we map two traits with mendelian inheritance: the major white spotting (S) locus and the hair ridge in Rhodesian ridgebacks. For both traits, we map the loci to discrete regions of <1 Mb. Fine-mapping of the S locus in two breeds refines the localization to a region of ~100 kb contained within the pigmentation-related gene MITF. Complete sequencing of the white and solid haplotypes identifies candidate regulatory mutations in the melanocyte-specific promoter of MITF. Our results show that genome-wide association mapping within dog breeds, followed by fine-mapping across multiple breeds, will be highly efficient and generally applicable to trait mapping, providing insights into canine and human health."
Evaluation of a proposed therapeutic protocol in 12 dogs with tentative degenerative myelopathy. Zoe S. Polizopoulou, Alexander F. Koutinas, Michael N. Patsikas, Nektarios Soubasis. Acta Vet. Hungarica. Sept. 2008;56(3):292-301. Quote: "The objective of this work was to evaluate the long-term efficacy of a proposed therapeutic protocol in 12 dogs with a tentative diagnosis of degenerative myelopathy, followed-up for a 6-month period. Twelve dogs fulfilling the antemortem inclusion criteria (breed, age, adequate vaccination, history of progressive posterior ataxia and/or paraparesis, no radiographic and myelographic abnormalities in the spinal cord and vertebral column) were allocated. All these dogs presented signs of thoracolumbar syndrome (T3-L3), scored as grade I (mild to moderate ataxia and paraparesis) in 10 and grade II (severe ataxia and ambulatory paraparesis) in 2 cases. Treatment included the use of ɛ-aminocaproic acid and N-acetylcysteine, supplemented with vitamins B, C and E. Prednisolone was given for the first two weeks and upon worsening of neurological signs. Daily exercise, performed as walking or swimming, was strongly recommended. Clinicopathological evaluation was normal in all 12 dogs, and survey radiographs and myelograms did not show spinal cord compression. Magnetic resonance imaging (MRI), performed only in 4 dogs, did not disclose compressive disorders or intramedullary lesions. Neurological signs were progressively worsening in all 12 animals, eventually resulting in severe paraparesis (grade III) or paraplegia (grade IV). The applied medications do not appear to be an attractive alternative to conservative management (physiotherapy) or euthanasia in canine degenerative myelopathy, irrespective of its chronicity."
Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Tomoyuki Awano, Gary S. Johnson, Claire M. Wade, Martin L. Katz, Gayle C. Johnson, Jeremy F. Taylor, Michele Perloski, Tara Biagi, Izabella Baranowska, Sam Long, Philip A. March, Natasha J. Olby, G. Diane Shelton, Shahnawaz Khan, Dennis P. O'Brien, Kerstin Lindblad-Toh, Joan R. Coates. PNAS. February 2009;106(8):2794-2799. Quote: "Canine degenerative myelopathy (DM) is a fatal neurodegenerative disease prevalent in several dog breeds. Typically, the initial progressive upper motor neuron spastic and general proprioceptive ataxia in the pelvic limbs occurs at 8 years of age or older. If euthanasia is delayed, the clinical signs will ascend, causing flaccid tetraparesis and other lower motor neuron signs. DNA samples from 38 DM-affected Pembroke Welsh corgi cases and 17 related clinically normal controls were used for genome-wide association mapping, which produced the strongest associations with markers on CFA31 in a region containing the canine SOD1 gene. SOD1 was considered a regional candidate gene because mutations in human SOD1 can cause amyotrophic lateral sclerosis (ALS), an adult-onset fatal paralytic neurodegenerative disease with both upper and lower motor neuron involvement. The resequencing of SOD1 in normal and affected dogs revealed a G to A transition, resulting in an E40K missense mutation. Homozygosity for the A allele was associated with DM in 5 dog breeds: Pembroke Welsh corgi, Boxer, Rhodesian ridgeback, German Shepherd dog, and Chesapeake Bay retriever. Microscopic examination of spinal cords from affected dogs revealed myelin and axon loss affecting the lateral white matter and neuronal cytoplasmic inclusions that bind anti-superoxide dismutase 1 antibodies. These inclusions are similar to those seen in spinal cord sections from ALS patients with SOD1 mutations. Our findings identify canine DM to be the first recognized spontaneously occurring animal model for ALS."
Degenerative myelopathy - diagnosis and treatment (Proceedings). Joan R. Coates. dvm360.com August 2009. Quote: "Canine degenerative myelopathy (DM) is a spontaneously occurring, adult-onset, progressive spinal cord disease. Degenerative myelopathy was first described by Averill as a spinal cord disorder that predominates in German Shepherd dogs. If decreased pelvic limb reflexes are observed, nerve root involvement is presumed and the disease termed chronic degenerative radiculomyelopathy. Initially thought to be specific to the GSD, it also was designated German Shepherd Dog myelopathy. This disease is not uncommon in some pure bred dogs with an overall prevalence rate of 0.19%. Although the German Shepherd Dog is the most commonly affected breed, DM has been reported in other breeds and most recently in the Pembroke Welsh Corgi (PWC). Higher disease prevalence has been determined in a number of other purebred dogs, such as the Boxer, Rhodesian Ridgeback, and Chesapeake Bay Retriever."
Prediction and Diagnosis of Canine Degenerative Myelopathy. Inventors: Joan R. Coates, Kerstin Lindblah-Toh, Claire Wade, Gary S. Johnson. US Patent Nr. 12/365,578. Sept. 2009. Quote: "The present invention provides for methods of identifying a dog carrying a major genetic risk factor in the SOD1 gene for degenerative myelopathy, a potential model for human amyeotrophic lateral sclerosis. Also provided a methods of early diagnosis, treatment and breeding based on the presence or absence of the marker."
Canine Degenerative Myelopathy. Joan R. Coates. Vet. Clinics of No. America. 2010. Quote: "Canine degenerative myelopathy (DM) was first described in 1973 by Averill as an insidious, progressive, general proprioceptive (GP) ataxia and upper motor neuron (UMN) spastic paresis of the pelvic limbs beginning in late adulthood, ultimately leading to paraplegia and necessitating euthanasia. Until recently, presence of primary axonal degeneration and nerve fiber loss that was restricted to spinal cord white matter and most severe in the mid to caudal thoracic region was compatible with a diagnosis of DM. The disease was termed "degenerative myelopathy" because of its histopathologic nature as a nonspecific degeneration of spinal cord tissue of undetermined cause. In 1975, Griffiths and Duncan published a series of cases with similar clinical signs and histologic changes in the white matter. They also reported hyporeflexia and nerve root involvement, and they termed the condition chronic degenerative radiculomyelopathy. Though most of the dogs in these initial reports were German Shepherd Dogs (GSD), other breeds were represented. Nonetheless, for many years, DM was considered an UMN and GP disease in the GSD. More recently DM has been recognized as a common problem in a number of breeds with an overall prevalence of 0.19%. Additionally, the clinical spectrum of DM has been broadened to involve both the UMN and lower motor neuron (LMN) systems. A recent advance in the molecular genetics of DM indicates that this canine disease may share pathogenic mechanisms with some forms of human amyotrophic lateral sclerosis (ALS - Lou Gehrig's disease)."
Development of a motor unit number estimation technique in normal dogs: a potential biomarker for canine degenerative myelopathy. Laura Vasquez. Univ. of Missouri. May 2011. Quote: "Motor unit number estimation (MUNE) is an electrophysiologic technique for quantifying the lower motor neuron (LMN) system. MUNE has proven useful in evaluating and monitoring neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). Recently a missense mutation in the canine superoxide dismutase 1 (SOD1) gene has been shown to be a risk factor for canine degenerative myelopathy (DM) suggesting homology to familial SOD1 ALS. To date, the LMN component of DM has not been well characterized or quantified. The modified incremental stimulation MUNE technique was applied to the sciatic-deep peroneal nerve branch with bilateral recordings at the extensor digitorum brevis muscle of 17 clinically normal dogs. Mean (± SD) value for the entire MUNE pool was 51 ± 21 with a range from 8 to 154. No statistically significant difference was noted between pelvic limbs (P=0.14) or between different age groups (< 7years old or >= 7 years old) (P=0.17). Test-retest reliability was assessed for trials performed under different anesthetic episodes (intermittent) versus trials performed under the same anesthetic episode (consecutive). The intraclass correlation coefficients for consecutive and intermittent MUNE evaluations were 0.73 and 0.65, respectively. These results provide preliminary reference ranges for normal dogs and document the potential utility of EDB modified incremental stimulation MUNE for longitudinal monitoring of lower motor neuron loss in DM affected dogs."
Degenerative Myelopathy in a Bernese Mountain Dog with a Novel SOD1 Missense Mutation. F.A. Wininger, R. Zeng, G.S. Johnson, M.L. Katz, G.C. Johnson, W.W. Bush, J.M. Jarboe, J.R. Coates. J Vet Intern Med. Sept. 2011;25:1166-1170.
The establishment of potential cerebrospinal fluid biomarkers for canine degenerative myelopathy. Shafie, Intan Nur Fatiha. Glasgow Theses Service. May 2013. Quote: "Canine degenerative myelopathy(DM) is a late onset neurodegenerative disease that primarily affects German Shepherd dog (GSD), though a number of other specific breeds are also affected. Other small breeds that have been reported with DM include Cavalier King Charles Spaniels. The underlying cause of the disorder remains elusive, though recent advances have implicated a mutation of superoxide dismutase 1(Sod1) in the aetiology, also implying DM is a potential orthologue of human amyotrophic lateral sclerosis. The identification of the Sod1 mutation raises the index of suspicion for an individual animal, however it is not specifically diagnostic as a proportion of dogs homozygous for the Sod1 mutation do not develop DM. Therefore, there is a clinical need for the development of specific biomarker(s) for DM to support genetic test. The aim of this study was to establish potential biomarkers for DM by exploring canine cerebrospinal fluid (CSF). A dual strategy was adopted;1) Evaluation of potential ALS biomarkers in DM CSF, 2) Identification of novel biomarker(s) in DM CSF. The cases selected in this project had a presumptive diagnosis of DM and were homozygous for Sod1 mutation [including one cavalier King Charles spaniel]. Preliminary characterisation by Western blot and mass spectrometry identified four protein candidates in DM CSF, comprised of cystatin C, transthyretin (dimeric and monomeric TTR), haptoglobin and clusterin. Since the validity of these putative biomarkers may be influenced by pre-analytical variables that may arise from the clinical environment, we therefore assessed the impact of three potential sample handling practices on these four proteins. The results from these experiments demonstrate that dimeric TTR and clusterin were affected by sample handling conditions. Therefore, an appropriate protocol for CSF sample handling was established. Western blot analyses indicated that clusterin is the most viable biomarker candidate for DM. Clusterin was significantly elevated in DM CSF when compared to a range of neurological conditions. The second potential candidate for DM biomarker is TTR, which is potentially reduced, an observation similar to those found in ALS CSF. The relationship of these proteins in the pathogenic mechanisms that underpin DM is unclear. However, based on observations on ALS, it is reasonable to speculate that their alterations are associated with a toxic gain of function of the mutant SOD1 protein. The successful characterisation of clusterin and TTR in DM CSF may therefore represent components of a panel of emerging biomarkers that may combine to distinguish DM in the clinic and provide further insights into the disease mechanisms."
Breed Distribution of SOD1 Alleles Previously Associated with Canine Degenerative Myelopathy. R. Zeng, J.R. Coates, G.C. Johnson, L. Hansen, T. Awano, A. Kolicheski, E. Ivansson, M. Perloski, K. Lindblad-Toh, D.P. O'Brien, J. Guo, M.L. Katz, G.S. Johnson. J Vet Intern Med. February 2014;28:515-521. Quote: "Background: Previous reports associated 2 mutant SOD1 alleles (SOD1:c.118A and SOD1:c.52T) with degenerative myelopathy in 6 canine breeds. The distribution of these alleles in other breeds has not been reported. Objective: To describe the distribution of SOD1:c.118A and SOD1:c.52T in 222 breeds. Animals: DNA from 33,747 dogs [including 73 cavalier King Charles spaniels] was genotyped at SOD1:c.118, SOD1:c.52, or both. Spinal cord sections from 249 of these dogs were examined. Methods: Retrospective analysis of 35,359 previously determined genotypes at SOD1:c.118G>A or SOD1:c.52A>T and prospective survey to update the clinical status of a subset of dogs from which samples were obtained with a relatively low ascertainment bias. Results: The SOD1:c.118A allele was found in cross-bred dogs and in 124 different canine breeds [including the CKCS] whereas the SOD1: c.52T allele was only found in Bernese Mountain Dogs. Most of the dogs with histopathologically confirmed degenerative myelopathy were SOD1:c.118A homozygotes, but 8 dogs with histopathologically confirmed degenerative myelopathy were SOD1:c.118A/G heterozygotes and had no other sequence variants in their SOD1 amino acid coding regions. The updated clinical conditions of dogs from which samples were obtained with a relatively low ascertainment bias suggest that SOD1: c.118A homozygotes are at a much higher risk of developing degenerative myelopathy than are SOD1:c.118A/G heterozygotes. [Of the 73 CKCSs, 10 (13.7%) were normal with no mutant allele; 27 (37%) were carriers of one of the mutant allele with a 50% chance of passing it on; and 36 (49.3%) had two of the mutant alleles and were at risk of developing signs of CDM and would pass the mutant allele on to its offspring.] Conclusions and Clinical Importance: We conclude that the SOD1:c.118A allele is widespread and common among privately owned dogs whereas the SOD1:c.52T allele is rare and appears to be limited to Bernese Mountain Dogs. We also conclude that breeding to avoid the production of SOD1:c.118A homozygotes is a rational strategy."
A retrospective study of the prevalence of the canine degenerative myelopathy associated superoxide dismutase 1 mutation (SOD1:c.118G > A) in a referral population of German Shepherd dogs from the UK. Angela L Holder, James A Price, Jamie P Adams, Holger A Volk and Brian Catchpole. Canine Genetics & Epidemiology. Sept. 2014. Quote: "Background: Canine degenerative myelopathy (CDM) is an adult onset, progressive neurodegenerative disease of the spinal cord. The disease was originally described in the German Shepherd dog (GSD), but it is now known to occur in many other dog breeds. A previous study has identified a mutation in the superoxide dismutase 1 gene (SOD1:c.118G > A) that is associated with susceptibility to CDM. In the present study, restriction fragment length polymorphism (RFLP) analysis was used to genotype GSD for SOD1:c.118G > A in order to estimate the prevalence of the mutation in a referral population of GSD in the UK. Results: This study demonstrated that the RFLP assay, based on use of PCR and subsequent digestion with the Eco571 enzyme, provided a simple genotyping test for the SOD1:c.118G > A mutation. In a young GSD population (i.e. dogs less than 6 years of age, before clinical signs of the disease usually become apparent), 8 of 50 dogs were found to be homozygous and a further 19 were heterozygous for the mutation. In dogs over 8 years of age, 21 of 50 dogs admitted to a tertiary referral hospital with pelvic limb ataxia as a major clinical sign were homozygous for the mutation, compared to none of 50 dogs of similar age, but where no neurological disease was reported on referral. Conclusions: This data suggests that genotyping for the SOD1:c.118G > A mutation is clinically applicable and that the mutation has a high degree of penetrance. Genotyping might also be useful for screening the GSD population to avoid mating of two carriers, but since the allele frequency is relatively high in the UK population of GSD, care should be taken to avoid reduction in genetic diversity within the breed."
Integrative Management of Degenerative Myelopathy. Angela Casey, Thomas Pfafman, Alexandra Mittner. Integrative Vet. Care J. 2015. Quote: A multifaceted treatment protocol is required for this multifaceted disease model. The greatest benefit is seen with early intervention. Case reports suggest that the progress of DM can be consistently slowed, halted or even temporarily reversed.
Variants within the SP110 nuclear body protein modify risk of canine degenerative myelopathy. Emma L. Ivansson, Kate Megquier, Sergey V. Kozyrev, Eva Muren, Izabella Baranowska Korberg, Ross Swofford, Michele Koltookian, Noriko Tonomura, Rong Zeng, Ana L. Kolicheski, Liz Hansen, Martin L. Katz, Gayle C. Johnson, Gary S. Johnson, Joan R. Coates, Kerstin Lindblad-Toh. PNAS. May 2016;113(22):E3091-3100. Quote: Canine degenerative myelopathy (DM) is a naturally occurring neurodegenerative disease with similarities to some forms of amyotrophic lateral sclerosis (ALS). ... We previously showed that DM is a promising model for ALS, because genome-wide association identified a mutation in superoxide dismutase 1 gene (SOD1), a known ALS gene. This mutation found in many dog breeds increases the risk of DM, and the pathological findings and clinical progression of the two diseases are similar. In this study, we identify a modifier gene, SP110 nuclear body protein (SP110), which strongly affects overall disease risk and age of onset in Pembroke Welsh Corgis at risk for DM. Dissecting the complex genetics of this disease in a model organism may lead to new insights about risk and progression in both canine and human patients. ... Most dogs that develop DM are homozygous for a common superoxide dismutase 1 gene (SOD1) mutation. However, not all dogs homozygous for this mutation develop disease. We performed a genome-wide association analysis in the Pembroke Welsh Corgi (PWC) breed comparing DM-affected and -unaffected dogs homozygous for the SOD1 mutation. The analysis revealed a modifier locus on canine chromosome 25. A haplotype within the SP110 nuclear body protein (SP110) was present in 40% of affected compared with 4% of unaffected dogs (P = 1.5 × 10−5), and was associated with increased probability of developing DM (P = 4.8 × 10−6) and earlier onset of disease (P = 1.7 × 10−5). SP110 is a nuclear body protein involved in the regulation of gene transcription. Our findings suggest that variations in SP110-mediated gene transcription may underlie, at least in part, the variability in risk for developing DM among PWCs that are homozygous for the disease-related SOD1 mutation. Further studies are warranted to clarify the effect of this modifier across dog breeds. ... The aim of the present study was to identify genetic modifiers of disease risk in dogs that are predisposed to DM by being homozygous for the SOD1 risk allele. We report that variants within SP110 modify the genetic risk and age of onset of DM in PWC dogs homozygous for mutant SOD1, and that those variants contribute to changes in the SP110 gene regulation and isoform ratio expressed in blood cells.
Frequency estimation of disease-causing mutations in the Belgian population of some dog breeds - Part 2: retrievers and other breed types. E. Beckers, M. Van Poucke, L. Ronsyn, L. Peelman. Vlaams Diergeneeskundiig Tijdschrift. October 2016:185-196. Quote: A Belgian population of ten breeds with a low to moderately low genetic diversity or which are relatively popular in Belgium, i.e. Bichon frise, Bloodhound, Bouvier des Flandres, Boxer, Cavalier King Charles spaniel, Irish setter, Papillon, Rottweiler, Golden retriever and Labrador retriever, was genotyped for all potentially relevant disease-causing variants known at the start of the study. In this way, the frequency was estimated for 26 variants in order to improve breeding advice. Disorders with a frequency high enough to recommend routine genotyping in breeding programs are (1) degenerative myelopathy for the Bloodhound, (2) arrhythmogenic right ventricular cardiomyopathy and degenerative myelopathy for Boxers, (3) episodic falling syndrome and macrothrombocytopenia for the Cavalier King Charles spaniel, (4) progressive retinal atrophy rod cone dysplasia 4 for the Irish setter (5) Golden retriever progressive retinal atrophy 1 for the Golden retriever and (6) exercise induced collapse and progressive rod-cone degeneration for the Labrador retriever. To the authors' knowledge, in this study, the presence of a causal mutation for a short tail in the Bouvier des Flandres is described for the first time. ... Duchenne muscular dystrophy (DMD-GR), a degenerative muscle disease, has an X-chromosome linked recessive mode of inheritance (Sharp et al., 1992). Other causal mutations have been described for the Cavalier King Charles spaniel (Walmsley et al., 2010) and the Rottweiler (Winand et al., 1994). The mutation was not found in the tested samples (n = 90) (Table 2). Nor did Broeckx et al. (2013) find the mutation in their population. No other frequency determinations have been performed as of yet.
Cerebrospinal Fluid Levels of Phosphorylated Neurofilament Heavy as a Diagnostic Marker of Canine Degenerative Myelopathy. C.M. Toedebusch, M.D. Bachrach, V.B. Garcia, G.C. Johnson, M.L. Katz, G. Shaw, J.R. Coates, M.L. Garcia. J. Vet. Int. Med. February 2017. Quote: Background: No definitive, antemortem diagnostic test for canine degenerative myelopathy (DM) is available. Phosphorylated neurofilament heavy (pNF-H) is a promising biomarker for nervous system diseases. Hypothesis/ Objective: Cerebrospinal fluid (CSF) and serum pNF-H is a detectable biological marker for diagnosis of canine DM. Animals: Fifty-three DM-affected, 27 neurologically normal, 7 asymptomatic at-risk, and 12 DM mimic dogs. Methods: Archived CSF and serum pNF-H concentrations were determined by a commercially available ELISA. A receiver-operating characteristic (ROC) curve was generated with CSF values. Results: Compared with old control dogs, median CSF pNF-H concentration was increased in all stages of DM; old dogs 5.1 ng/mL (interquartile range [IQR] 1.4-9.3) versus DM stage 1 23.9 ng/mL (IQR 20.8-29.6; P < .05) versus DM stage 2 36.8 ng/mL (IQR 22.9-51.2; P < .0001) versus DM stage 3 25.2 ng/mL (IQR 20.2-61.8; P < .001) versus DM stage 4 38.0 ng/mL (IQR 11.6-59.9; P < .01). Degenerative myelopathy stage 1 dogs had increased median CSF pNF-H concentrations compared with asymptomatic, at-risk dogs (3.4 ng/mL [IQR 1.5-10.9; P < .01]) and DM mimics (6.6 ng/mL [IQR 3.0-12.3; P < .01]). CSF pNF-H concentration >20.25 ng/mL was 80.4% sensitive (confidence interval [CI] 66.09-90.64%) and 93.6% specific (CI 78.58-99.21%) for DM. Area under the ROC curve was 0.9467 (CI 0.92-0.9974). No differences in serum pNF-H concentration were found between control and DM-affected dogs. Conclusions and Clinical Importance: pNF-H concentration in CSF is a sensitive biomarker for diagnosis of DM. Although there was high specificity for DM in this cohort, further study should focus on a larger cohort of DM mimics, particularly other central and peripheral axonopathies.
Retrospective Study of 240 Dogs Receiving Gabapentin for Chronic Pain Relief. Lily V Davis, Peter W Hellyer, Robin A Downing, Lori R Kogan. J. Vet.Med. & Research. August 2020;7(4):1194-1197. Quote: Our goal was to assess gabapentin dosage and tolerability in dogs taking it for chronic pain. ... All patients in this study were on gabapentin due to an inability to fully control their pain with NSAIDs or nutraceuticals alone. ... The majority (217 patients; 90.42%) had pain localized to their back. The other anatomic locations of pain included stifle (33; 13.8%), elbow/shoulder (21; 8.8%) and hip (11; 4.6%). Some patients' pain was localized to multiple areas and is counted in all categories. The most common diagnosis of pain was osteoarthritis (203; 84.6%), followed by generalized, nonspecific back pain (22;9.2%), intervertebral disk disease (18; 7.5%), and degenerative myelopathy (11; 4.6%). Some patients experienced two different kinds of pain and are embodied in both categories. One hundred and thirty-six (56.67%) patients did not have definitive anatomic cause for their pain, such as cruciate tears or intervertebral disc disease, as seen in Table 2. ... We retrospectively analyzed the medical records of 240 dogs taking gabapentin for chronic pain and systematically assessed: patient signalment, definitive diagnosis, location and description of pain, VAS scores immediately preceding and following the patient's maximum gabapentin dose, maximum gabapentin dosage, presence or absence of side effects related to gabapentin use, use of NSAID/immunomodulator drugs and nutraceuticals, presence or absence of levothyroxine supplementation, surgical procedures, and physical medicine. The range of tolerated gabapentin doses was 6.9 - 500 mg/kg/day [3.1- 227.3 mg/lb], PO, q12 hr (every 12 hrs) and only 10% of patients experienced the most common side effect of sedation. ... The results from this case series suggest that gabapentin is well-tolerated at much higher doses than what is typically prescribed. Side effects were uncommon, with no clear pattern based on dose, or dog size or age. Therefore, like many other analgesic medications, the efficacy of gabapentin appears patient-specific and should be dosed to effect until side effects are noted or analgesia is achieved.
The Long-Term Clinical Course of Canine Degenerative Myelopathy and Therapeutic Potential of Curcumin. Yui Kobatake, Kohei Nakata, Hiroki Sakai, Jun Sasaki , Osamu Yamato, Satoshi Takashima, Naohito Nishii, Sadatoshi Maeda, Md Shafiqul Islam, Hiroaki Kamishina. Vet. Sci. September 2021; doi: 10.3390/vetsci8090192. Quote: Canine degenerative myelopathy (DM), recognized as a spontaneous model of amyotrophic lateral sclerosis, is known as a late-onset progressive degenerative disease of the spinal cord. Because of the progressive nature of DM, many dogs are elected to be euthanized, resulting in limited information on the end-stage clinical presentation. We investigated the long-term clinical course from diagnosis to natural death to further deepen our understanding of the entire clinical picture of this disease. Because curcumin was administered in some cases, the therapeutic effect of curcumin on DM was also examined. Forty [40] dogs included in this study were client-owned Pembroke Welsh Corgis with a definitive diagnosis of DM by necropsy and histopathology. Dogs were excluded from this study if they died from another disease or were elected to be euthanized. Information on the long-term clinical symptoms of DM was investigated based on a questionnaire, which was collected from the dog owners. Urinary incontinence and respiratory disorder were observed in most dogs, as was respiratory impairment-correlated death. In contrast, signs consistent with brainstem dysfunction were noticed at the terminal stage in a small portion of dogs. ... Dogs in the curcumin-treated group included one intact and four neutered males and one intact and two spayed females. No adverse events associated with treatment were observed throughout the study. The dogs treated with curcumin had a significantly longer survival time than those who had not received treatment. ... The timing of the onset of thoracic limb paresis was not significantly different between the curcumin and control groups; however, nonweight bearing of the hind/thoracic limbs was prolonged in the curcumin-treated dogs when compared with the control dogs. Owing to the small number of cases presenting clinical symptoms other than gait disturbance, analyzing whether there was a difference between the curcumin-treated and control groups was not possible. However, as respiratory disorder was a common symptom, we compared the timing of respiratory disorder onset between the two groups but found no significant difference. ... We found that dogs with DM treated with curcumin had a significantly longer survival time than those who did not receive this treatment. Curcumin is a biologically potent polyphenolic compound that has various effects on the central nervous system, including neuroprotective effects such as antioxidant and anti-inflammatory activities. Furthermore, a previous study suggested that curcumin molecules bound strongly to the aggregation-prone regions of the mutant SOD1 proteins and blocked the exposed aggregation site, leading to the inhibition of the formation of SOD1 unstructured aggregates. In addition, there are three known beneficial effects of curcumin on muscles: (1) promotion of protein synthesis; (2) reduction of muscle protein degradation; and (3) reduction of exercise muscle injuries. There were no statistically significant differences in the duration from onset to the paresis of thoracic limbs in the curcumin and control groups. Nevertheless, the duration from onset to the nonweight bearing of hind/thoracic limbs was longer in dogs from the curcumin group. This suggests that curcumin may be efficient in preventing neurodegeneration and muscle atrophy in dogs with DM. Overall, the results of this study suggest that the administration of curcumin has a neuroprotective and muscle-protective potential against DM, and perhaps against ALS as well. ... This study has improved the characterization of the end-stage clinical presentation of DM in dogs, particularly urinary and fecal incontinence, brainstem disorders, and dyspnea. Dyspnea may be related to the death of dogs with DM as well as that of patients with ALS. The characterization of longitudinal changes in the clinical presentation of DM will assist veterinary practitioners and owners in recognizing when and how to provide appropriate care to DM-affected dogs and will be helpful in determining target therapies. ... Although further studies with more cases are needed, the results of this study suggest that administration of curcumin is effective in slowing the progression of DM.
Diagnosis and management of dogs with degenerative myelopathy: A survey of neurologists and rehabilitation professionals. Teryn V. Bouche, Joan R. Coates, Sarah A. Moore, Dominik Faissler, Mark Rishniw, Natasha J. Olby. J. Vet. Intern. Med. August 2023; doi: 10.1111/jvim.16829. Quote: Background: Antemortem diagnosis of degenerative myelopathy (DM) in dogs is presumptive and there are no accepted guidelines for the management of this condition. Hypothesis/ Objectives: Describe current practices of neurology clinicians and physical rehabilitation professionals in the diagnosis and management of DM. Animals: None. Methods: Online surveys examining diagnosis and management of DM were constructed and distributed via neurology and rehabilitation listservs. Results: One hundred ninety neurology and 79 rehabilitation professionals from 20 countries participated. Most neurology (142/189) and rehabilitation (23/39) respondents required genetic testing for the superoxide dismutase 1 (SOD1) mutation and 82/189 neurologists also required spinal magnetic resonance imaging (MRI) for presumptive DM diagnosis. Most neurology respondents recommended exercise (187/190) and physical rehabilitation (184/190). Over 50% (102/190) of neurology respondents perform rechecks on dogs diagnosed with DM. Rehabilitation respondents reported preservation or improvement of strength (78/79) and coordination (77/79) as therapeutic goals. At-home exercises (75/79), underwater treadmill (64/79), gait training (55/79), and strength building exercises (65/79) were used to maintain strength (58/79), coordination (56/79), muscle mass (56/79), and improve overall wellbeing (54/79). Neurology respondents reported that owners elect euthanasia when dogs become nonambulatory paraparetic whereas rehabilitation respondents report euthanasia when paraplegia and incontinence develop. Conclusion and Clinical Importance: The majority of dogs diagnosed with DM have not undergone advanced imaging, the combination of history, neurological findings, and genetic testing is heavily relied upon. Whereas the diagnosis of DM is frequently made by veterinary neurologists, continued care is often performed by rehabilitation professionals or primary veterinarians.


CONNECT WITH US