Diabetes Mellitus and Cavalier King Charles Spaniels
-
 What It Is
What It Is - Symptoms
- Diagnosis
- Related Disorders
- Treatment
- -- dietary care
- Breeders' Responsibilities
- Research News
- Related Links
- Veterinary Resources
The cavalier King Charles spaniel is genetically susceptible to developing an incurable disease known as diabetes mellitus, and cavaliers are at a greater risk of developing diabetes mellitus than many other breeds. *
* See Canine Inherited Disorders Database.
What It Is
Diabetes mellitus (DM) is commonly called "sugar diabetes" (also "type 1 diabetes"), because it results in excessively high levels of blood sugar, and the presence of sugar (glucose) in the urine. The disease prevents the cavalier from converting the glucose in its diet into energy. It is caused by a deficiency of the hormone insulin. Insulin is required by muscles, fat tissue, and the liver to utilize carbohydrates, proteins, and fats ingested in the diet.
The average age of dogs that develop diabetes mellitus is 6 to 8 years. However, cavaliers as young as six months of age have become diabetic and have remained so for the rest of their lives.
In a 2007 study, UK researchers found that the CKCS "showed 8 positive associations for [an IL-10 haplotype which is associated with diabetes], all of which had OR [odds ratio] between 3.30 and 4.05. Such a high number of associated alleles in this gene were indicative of a risk haplotype for this breed. IL-10 did not show any associations for any other breed in this analysis." In a 2009 follow-up study, the same research team found that:
"when SNPs were examined for an association with diabetes ... significant associations were observed for ... IL-10 in the Cavalier King Charles Spaniel. These results suggest that canine cytokine genes regulating the TH1/TH2 immune balance might play a contributory role in determining susceptibility to diabetes in [the CKCS]."
In a July 2014 study which included 107 cavaliers, the researchers concluded that "canine diabetes is a heterogeneous condition and is most likely to be a polygenic trait in most dog breeds."
In a May 2023 article, UK researchers found that the treatment of cavaliers with glucocorticoids (steroid hormones that veterinarians frequently administer to dogs to reduce inflammation and suppress the immune system) may increase the breed's risk of developing DM. They compared dogs diagnosed with DM to treatments with systemic glucocorticords within the preceding 6 weeks. They found that cavaliers were the third most common breed (behind West Highland white terrriers and Tibetan terriers) which had been exposed to glucocorticord treatments within 6 weeks prior to being diagnosed with DM. They concluded that "Exposure to glucocorticoids is associated with a substantial increase in the risk of developing DM for the dog breeds included in this analysis."
RETURN TO TOP
Symptoms
Symptoms of the disease include:
• a grossly exaggerated thirst
• a poor appetite
• weight loss
• a sweet smell on the breath.
If the diabetic cavalier is not treated soon enough and correctly, it can develop far more serious form of diabetes mellitus, called ketoacidosis, with symptoms including:
• sudden onset of cataracts in the eyes
• lethargy
• vomiting
• diarrhea
• blindness due to degeneration of the retina
• coma.
Ketoacidosis represents a very serious disruption to the dog's metabolism and will most likely result in death if left untreated.
RETURN TO TOP
Diagnosis
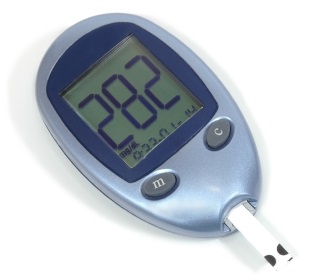
Diabetes mellitus is diagnosed on the basis of the dog's symptoms and an abnormally high blood glucose measurement. The normal level of glucose in the blood is 4 to 7 mmol/L. Diabetes mellitus is the only common disease which will cause blood glucose levels to rise above and stay above that normal level.
RETURN TO TOP
Related Disorders
Diabetes insipidus (DI) is the other major form of diabetes in dogs. It describes the passage of large volumes of very diluted urine. Its possible causes are (a) the failue of the production of hte antidiuretic hormone (ADH), vasopressin, by the dog's hypothalamus, or (b) the failure of the kidneys to respond properly to vasopressin. Vasopressin is a hormone produced by the pituitary gland, which serves to regulate urine production by the kidneys and the amount of water that the body retains . Desmopressin (DDAVP, Stimate) is the primary drug for treating DI. It is a synthetic replacement for vasopressin.
In a January 2015 report, UK author Lucy J. Davison has observed that cases of exocrine pancreatic inflammation often accompany diabetes mellitus. She states:
"However, the question remains as to whether the diabetes mellitus causes the pancreatitis or whether, conversely, the pancreatitis leads to diabetes mellitus – as there is evidence to support both scenarios. The concurrence of diabetes mellitus and pancreatitis has clinical implications for case management as such cases may follow a more difficult clinical course, with their glycaemic control being 'brittle' as a result of variation in the degree of pancreatic inflammation. Problems may also arise if abdominal pain or vomiting lead to anorexia. In addition, diabetic cases with pancreatitis are at risk of developing exocrine pancreatic insufficiency in the following months to years, which can complicate their management further."
In an April 2014 study, Portugese veterinarians, searching the genetics of canine periodontal disease, discovered that variants of the interleukin-10 gene, particularly interleukin-10 (IL-10), is "highly polymorphic with genetic variants that may be important in PD susceptibility." As noted above, studies of IL-10 in the cavalier King Charles Spaniel suggest that they may play a role in determining the cavaliers' susceptibility to diabetes.
RETURN TO TOP
Treatment
 Diabetes mellitus cannot be cured.
The goals of treatment are to minimize the clinical symptoms and prevent
complications (such as hypoglycemia [low blood sugar] and ketoacidosis).
It is medically managed with insulin and diet.
Diabetes mellitus cannot be cured.
The goals of treatment are to minimize the clinical symptoms and prevent
complications (such as hypoglycemia [low blood sugar] and ketoacidosis).
It is medically managed with insulin and diet.
The cavalier will require daily, carefully measured and administered injections of insulin for its entire life. The administration of insulin is the most important aspect of controlling diabetes mellitus. Most dogs require only one injection of insulin per day, though two may be needed. Drugs in tablet form which stimulate the pancreas to produce more insulin have proven to be ineffective in controlling diabetes in dogs.
In addition to injecting the insulin daily, the owner of the diabetic cavalier will need to closely monitor the dog to ensure its diabetes is under control. This may require that the owner collect and test urine samples daily to determine the presence of glucose and adjust the dose of insulin accordingly. Blood glucose monitoring is another way of determining the dosage of insulin.
Diet management will be important to minimize the impact of food on the dog's glycemic response. So, carbohydrates and fat content likely will be reduced and fiber content increased. Meals will have to be scheduled daily to coincide with the timing of insulin injections. Diabetic dogs are predisposed to dehydration and need access to fresh, clean water all day.
In a February 2013 report in Diabetes, researchers have shown that it is possible to cure diabetes in dogs with a single session of gene therapy. After a single gene therapy session, the dogs reportedly recover their health and no longer show symptoms of the disease. In some cases, monitoring continued for over four years, with no recurrence of symptoms. The therapy is minimally invasive; it consists of a single session of various injections in the dog's rear legs. These injections introduce gene therapy vectors, with a dual objective: to express the insulin gene, on the one hand, and that of glucokinase, on the other. Glucokinase is an enzyme that regulates the uptake of glucose from the blood. When both genes act simultaneously they function as a "glucose sensor", which automatically regulates the uptake of glucose from the blood, thus reducing diabetic hyperglycemia (the excess of blood sugar associated with the disease). For more information, click here.
-- dietary care
Some older studies have indicated that high-fiber diets might improve glycaemic control in diabetic dogs. See, for example, this February 2002 article.
More recent investigations have found no clear clinical benefit for diabetic dogs being fed high-fiber formulations compared with being fed conventional adult maintenance diets. A Novermber 2009 article compared the traditional diabetes recipes of high in fiber and moderate in carbohydrates with a commercial diet with moderate-fiber, low-carbohydrate, and higher fat. The researchers reported no significant differences in insulin requirement or glycaemic control among the diets. They observed that weight loss occurred when the dogs were fed the traditional diabetes diet, while weight was maintained with the other two diets. They concluded that, for stable diabetic dogs, high-fiber, moderate carbohydrate diets offered no significant advantage compared with a commercial diet with moderate fiber and low carbohydrate. They recommended that diets with high fiber, moderate carbohydrate, and moderate fat should not be routinely recommended for diabetic ogs with thin body conditions.
In an August 2018 article, a team of Brazilian veterinarians studied fifteen dogs suffering from diabetes mellitus (DM) by comparing the effects of a pea-&-barley diet with a corn diet upon glycaemic control. Both diets were in dry kibble form and otherwise contained similar ingredients. An implanted continuous glucose monitoring system (CGMS) was used to measure blood glucose levels. The same insulin dose was maintained for each dog throughout the study. The researchers report finding that:
"According to the results obtained from this group of fifteen animals under the experimental design used here, diabetic dogs receiving a diet with peas and barley as the starch source resulted in lesser variation of interstitial and plasma glucose concentration than the period receiving a maize-based diet. Therefore, processing applied to a maize-based diet was not enough to result in the same effects of a peas- and barley-based diet on glycaemic control in diabetic dogs."
In his August 2015 review article, Dr. Stijn Niessen of the Royal Veterinary College in the UK, noted that, despite the wide range of diabetic dog diets available, it remains to be proven that such diets, which often have a high fiber content, offer a clinically significant advantage. He stated:
"In fact, weight loss occurred when the dogs were fed the high-fibre, moderate-carbohydrate, moderate-fat diet, which is anything but desirable in many diabetic dogs, which suffer from pathological weight loss and therefore a poor body condition score to start with. The author therefore determines the type of chosen diet mainly on the basis of presence of any co-morbidity (not the diabetes itself, e.g. pancreatitis, inflammatory bowel disease or renal disease), and especially the palatability of the food for the individual patient. Since we are trying to mimic the lost function of the endocrine pancreas, and we therefore want to match the hypoglycaemic action of the twice-daily insulin injections with the hyperglycemic effect of the twice-daily meals, palatability is absolutely crucial, ensuring reliable uptake of a steady amount of calories at regular intervals. Many good-quality commercial diets can offer this."
Beware of veterinarians treating your dog for diabetes, who seem to instinctively recommend a "prescription diet" said to be designed for diabetic dogs. Among such diets are dry and canned wet versions of Hill's w/d. These products are typical of the high fiber diets with significantly more carbohydrate than protein. The dry Hill's w/d also is loaded with grains and very weak on protein. It's top ingredients are "Whole Grain Wheat, Whole Grain Corn, Powdered Cellulose, Chicken Meal, Corn Gluten Meal, Whole Grain Sorghum, Soybean Mill Run", which alone should convince most readers to want to avoid feeding it to their dogs, if at all possible. It consists of 46.1% fiber, 50.7% carbohydrate (which also includes some of the fiber), 9.1% fat, and only 18.9% protein.
Based upon the above-cited more current research, the best diet for most diabetic dogs would be a canned food which is relatively high in protein and low in carbohydrate, with only moderate fiber. However, as Dr. Niessen noted, if the diabetic dog is over-weight or also suffers from a related disorder, such as pancreatitis, the diet may have to take that factor into account as well.
RETURN TO TOP
Breeders' Responsibilities
 The
Canine Inherited Disorders Database recommends that dogs with symptoms of
diabetes mellitus should not be bred, and parents and siblings should be
considered potential carriers. As it is considered a likely genetic disease in
Cavaliers, all breeding stock should be tested for consistently high blood
glucose levels. Any littermates of breeding stock having diabetes mellitus
should be taken into consideration. Unfortunately, since the disease is known to
not develop until as late as 8 years, breeding stock which may test clear for
symptoms of diabetes mellitus may well carry the genes which cause it and may
themselves develop symptoms of the disease later in life. Therefore, there is no
certain test to assure that any cavalier breeding stock is clear of diabetes
mellitus.
The
Canine Inherited Disorders Database recommends that dogs with symptoms of
diabetes mellitus should not be bred, and parents and siblings should be
considered potential carriers. As it is considered a likely genetic disease in
Cavaliers, all breeding stock should be tested for consistently high blood
glucose levels. Any littermates of breeding stock having diabetes mellitus
should be taken into consideration. Unfortunately, since the disease is known to
not develop until as late as 8 years, breeding stock which may test clear for
symptoms of diabetes mellitus may well carry the genes which cause it and may
themselves develop symptoms of the disease later in life. Therefore, there is no
certain test to assure that any cavalier breeding stock is clear of diabetes
mellitus.
RETURN TO TOP
Research News
May 2023:
Cavaliers ranked third in developing diabetes mellitus soon
after being treated with steroids.
 In
a
May 2023 article, UK researchers Angela M. Heeley, Dave C. Brodbelt
(right), Dan G. O’Neill, David B. Church, and Lucy J. Davison
compared dogs diagnosed with diabetes mellitus (DM) to treatments with
systemic glucocorticords within the preceding 6 weeks. Glucocorticoids
are a type of steroid hormone that veterinarians frequently administer
to dogs to reduce inflammation and suppress the immune system. The
researchers noted that case studies have reported
glucocorticoid-associated hyperglycaemia and DM in dogs in individual
cases. They examined the records of 14 breeds of dogs which had 10 or
more cases of DM during 2016 and 2017, including cavalier King Charles
spaniels. They found that cavaliers were the third most common breed
(behind West Highland white terrriers and Tibetan terriers) which had
been exposed to glucocorticord treatments within 6 weeks prior to being
diagnosed with DM. They concluded that "Exposure to glucocorticoids is
associated with a substantial increase in the risk of developing DM for
the dog breeds included in this analysis."
In
a
May 2023 article, UK researchers Angela M. Heeley, Dave C. Brodbelt
(right), Dan G. O’Neill, David B. Church, and Lucy J. Davison
compared dogs diagnosed with diabetes mellitus (DM) to treatments with
systemic glucocorticords within the preceding 6 weeks. Glucocorticoids
are a type of steroid hormone that veterinarians frequently administer
to dogs to reduce inflammation and suppress the immune system. The
researchers noted that case studies have reported
glucocorticoid-associated hyperglycaemia and DM in dogs in individual
cases. They examined the records of 14 breeds of dogs which had 10 or
more cases of DM during 2016 and 2017, including cavalier King Charles
spaniels. They found that cavaliers were the third most common breed
(behind West Highland white terrriers and Tibetan terriers) which had
been exposed to glucocorticord treatments within 6 weeks prior to being
diagnosed with DM. They concluded that "Exposure to glucocorticoids is
associated with a substantial increase in the risk of developing DM for
the dog breeds included in this analysis."
February 2020:
Cavaliers rank ninth in prevalence of having diabetes mellitus
among Australian dogs.
 In
a
February 2020 article, a team of Australian researchers (Samuel
Yoon, Linda M Fleeman, Bethany J Wilson, Caroline S Mansfield, Paul
McGreevy [right]) examined 134,329 records of dogs treated in
152 veterinary clinics in Australia in 2017, finding 418 dogs diagnosed
with diabetes mellitus (DM). The breeds with the highest number of DM
cases were: Australian terriers, Siberian huskies, English springer
spaniels, West Highland white terriers, miniature schnauzers, poodles,
Bichon Frises, schnauzers, and cavalier King Charles spaniels (ranked
ninth). They also found that females are more predisposed to DM than
males, and that neutered males were at increased risk for DM compared to
intact males and neutered females. See also this
September 2019 item below.
In
a
February 2020 article, a team of Australian researchers (Samuel
Yoon, Linda M Fleeman, Bethany J Wilson, Caroline S Mansfield, Paul
McGreevy [right]) examined 134,329 records of dogs treated in
152 veterinary clinics in Australia in 2017, finding 418 dogs diagnosed
with diabetes mellitus (DM). The breeds with the highest number of DM
cases were: Australian terriers, Siberian huskies, English springer
spaniels, West Highland white terriers, miniature schnauzers, poodles,
Bichon Frises, schnauzers, and cavalier King Charles spaniels (ranked
ninth). They also found that females are more predisposed to DM than
males, and that neutered males were at increased risk for DM compared to
intact males and neutered females. See also this
September 2019 item below.
September 2019: Cavaliers were among breeds having higher odds of having diabetes mellitus in Australia in 2017. In a June 2019 abstract presented at the 2019 ACVIM Forum, Australian veterinary researchers (Linda M. Fleeman, Samuel Yoon, Bethany Wilson, Caroline Mansfield, Paul McGreevy) reviewed the medical records of 134,329 dogs treated in Australia in 2017, finding 481 of them diagnosed with diabetes mellitus (DM), a species-wide prevalence of 0.36%. Cavalier King Charles spaniels were among nine breeds having "significanly higher odds of having DM. See also this February 2020 item above.
September 2018:
Brazilian researchers report that diabetic dogs had
lower blood glucose levels on diet of peas and barley instead of corn as
main starch source.
 In an
August 2018 article, a team of Brazilian veterinarians (Fabio
A. Teixeira [right], Daniela P. Machado, Juliana T. Jeremias, Mariana R.
Queiroz, Cristiana F. F. Pontieri, Marcio A. Brunetto) studied fifteen
dogs suffering from diabetes mellitus (DM) by comparing the effects of a
pea-&-barley diet with a corn diet upon glycaemic control. Both diets
were in dry kibble form and otherwise contained similar ingredients. An
implanted continuous glucose monitoring system (CGMS) was used to
measure blood glucose levels. The same insulin dose was maintained for
each dog throughout the study. The researchers report finding that:
In an
August 2018 article, a team of Brazilian veterinarians (Fabio
A. Teixeira [right], Daniela P. Machado, Juliana T. Jeremias, Mariana R.
Queiroz, Cristiana F. F. Pontieri, Marcio A. Brunetto) studied fifteen
dogs suffering from diabetes mellitus (DM) by comparing the effects of a
pea-&-barley diet with a corn diet upon glycaemic control. Both diets
were in dry kibble form and otherwise contained similar ingredients. An
implanted continuous glucose monitoring system (CGMS) was used to
measure blood glucose levels. The same insulin dose was maintained for
each dog throughout the study. The researchers report finding that:
"According to the results obtained from this group of fifteen animals under the experimental design used here, diabetic dogs receiving a diet with peas and barley as the starch source resulted in lesser variation of interstitial and plasma glucose concentration than the period receiving a maize-based diet. Therefore, processing applied to a maize-based diet was not enough to result in the same effects of a peas- and barley-based diet on glycaemic control in diabetic dogs."
August 2015: Extreme beta-cell deficiency is noted in diabetic dogs. In a June 2015 report, US researchers (Emily J. Shields, Carol J. Lam, Aaron R. Cox, Matthew M. Rankin, Thomas J. Van Winkle, Rebecka S. Hess, Jake A. Kushner) announce finding an extreme loss of β-cells in dogs affected with diabetes.
January 2015:
Which disorder causes the other? Diabetes mellitus or exocrine pancreatic
inflammation?
 In a
January 2015 report, UK author Lucy
J. Davison (right) has observed
that cases of diabetes mellitus often accompany
exocrine pancreatic
inflammation. She states:
In a
January 2015 report, UK author Lucy
J. Davison (right) has observed
that cases of diabetes mellitus often accompany
exocrine pancreatic
inflammation. She states:
"However, the question remains as to whether the diabetes mellitus causes the pancreatitis or whether, conversely, the pancreatitis leads to diabetes mellitus – as there is evidence to support both scenarios. The concurrence of diabetes mellitus and pancreatitis has clinical implications for case management as such cases may follow a more difficult clinical course, with their glycaemic control being 'brittle' as a result of variation in the degree of pancreatic inflammation. Problems may also arise if abdominal pain or vomiting lead to anorexia. In addition, diabetic cases with pancreatitis are at risk of developing exocrine pancreatic insufficiency in the following months to years, which can complicate their management further."
 October
2013:
PennVet
researchers find glargine insulin is "peakless" and maintains blood glucose
concentration. In an
October 2013 report, University of Pennsylvania veterinary researchers
Rebecka S. Hess and Kenneth J. Drobatz found
that, in ten dogs with diabetes mellitus "fed a diet high in insoluble fiber,
glargine insulin is a peakless insulin that does not induce a distinct blood
glucose concentration nadir." (See
October
2012 item below.)
October
2013:
PennVet
researchers find glargine insulin is "peakless" and maintains blood glucose
concentration. In an
October 2013 report, University of Pennsylvania veterinary researchers
Rebecka S. Hess and Kenneth J. Drobatz found
that, in ten dogs with diabetes mellitus "fed a diet high in insoluble fiber,
glargine insulin is a peakless insulin that does not induce a distinct blood
glucose concentration nadir." (See
October
2012 item below.)
February 2013:
Gene therapy
reportedly cures Type 1 diabetes in dogs. Researchers at the
Universitat Autònoma de Barcelona (UAB) in Spain, led by Fàtima Bosch
(right),
in
a February 2013 report in
 Diabetes, have shown for the first time that it is
possible to cure diabetes in dogs with a single session of gene therapy. After a
single gene therapy session, the dogs recover their health and no longer show
symptoms of the disease. In some cases, monitoring continued for over four
years, with no recurrence of symptoms. The therapy is minimally invasive; it
consists of a single session of various injections in the dog's rear legs. These
injections introduce gene therapy vectors, with a dual objective: to express the
insulin gene, on the one hand, and that of glucokinase, on the other.
Glucokinase is an enzyme that regulates the uptake of glucose from the blood.
When both genes act simultaneously they function as a "glucose sensor", which
automatically regulates the uptake of glucose from the blood, thus reducing
diabetic hyperglycemia (the excess of blood sugar associated with the disease).
For more information,
click here.
Diabetes, have shown for the first time that it is
possible to cure diabetes in dogs with a single session of gene therapy. After a
single gene therapy session, the dogs recover their health and no longer show
symptoms of the disease. In some cases, monitoring continued for over four
years, with no recurrence of symptoms. The therapy is minimally invasive; it
consists of a single session of various injections in the dog's rear legs. These
injections introduce gene therapy vectors, with a dual objective: to express the
insulin gene, on the one hand, and that of glucokinase, on the other.
Glucokinase is an enzyme that regulates the uptake of glucose from the blood.
When both genes act simultaneously they function as a "glucose sensor", which
automatically regulates the uptake of glucose from the blood, thus reducing
diabetic hyperglycemia (the excess of blood sugar associated with the disease).
For more information,
click here.
October
2012:
Penn Vet
School seeks diabetic dogs for new insulin study.
 Dr.
Rebecka Hess (right), chief of the Section of Medicine at the University of
Pennsylvania's School of Veterinary Medicine is conducting a clinical trial, for
which she is currently recruiting dogs with well-regulated diabetes, which aims
to test how well a strategy commonly used to treat humans with diabetes will aid
in better controlling the disease in canines. Dogs in the trial will be given a
very short-acting insulin, Lispro, to the dogs' current NPH
insulin (neutral protamine Hagedorn) treatment regimen. Dogs must be on a
current NPH insulin regimen and must be fed Hill's w/d Prescription Diet*.
No change in lifestyle, cost, or schedule will be needed. Study participation
will be free of charge, and Lispro insulin, blood work, and urine testing will
also be free. (See also,
October 2013
item above.)
Dr.
Rebecka Hess (right), chief of the Section of Medicine at the University of
Pennsylvania's School of Veterinary Medicine is conducting a clinical trial, for
which she is currently recruiting dogs with well-regulated diabetes, which aims
to test how well a strategy commonly used to treat humans with diabetes will aid
in better controlling the disease in canines. Dogs in the trial will be given a
very short-acting insulin, Lispro, to the dogs' current NPH
insulin (neutral protamine Hagedorn) treatment regimen. Dogs must be on a
current NPH insulin regimen and must be fed Hill's w/d Prescription Diet*.
No change in lifestyle, cost, or schedule will be needed. Study participation
will be free of charge, and Lispro insulin, blood work, and urine testing will
also be free. (See also,
October 2013
item above.)
* EDITOR'S NOTE: Hill's w/d Prescription Diet dry dog food is junk, by the way. It contains NO meat whatsoever. The canned version barely has any meat and relies primarily on corn filler and egg as the protein sources. If only researchers had a better sense of the importance of healthful, fresh meats and vegetables in studies such as this one. See our Diets page for more insight into the severe limitations of the knowledge of many veterinarians about canine dietary needs.
July 2012: Insulin pen is now available for diabetic dogs. MSD Animal Health has introduced Caninsulin VetPen, the first insulin pen specifically designed for diabetic dogs and cats. VetPen reportedly simplifies dosing and administration of insulin. An insulin pen is used to inject insulin for the treatment of diabetes. It is composed of an insulin cartridge (integrated or bought separately) and a dial to measure the dose, and is used with disposable pen needles to deliver the dose. For more information about Caninsulin, click here.
November 2010: Researchers find that a new topical product, Kinostat, can significantly delay the onset and/or progression of cataracts in dogs with diabetes mellitus. Read more here.
RETURN TO TOP
Related Links
RETURN TO TOP
Veterinary Resources
Control of Canine Genetic Diseases. Padgett, G.A., Howell Book House 1998, pp. 198-199, 232.
Dogs, Diet, and Disease: An Owner’s Guide to Diabetes Mellitus, Pancreatitis, Cushing’s Disease, and More. Caroline D. Levin RN. Lantern Publ. 2001.
Management of Canine Diabetes. Linda M. Fleeman, Jacqueline S. Rand. Vet. Clinics of No. Amer.: Small Anim. Pract. September 2001;31(5):855-880. Quote: Most diabetic dogs seem to have a late-onset form of type 1 diabetes analogous to latent autoimmune diabetes of adults in human beings, which is characterized by absolute insulin deficiency. The three goals of therapy for diabetic dogs are resolution of all clinical signs, avoidance of insulin-induced hypoglycemia, and resumption of their usual lifestyle and exercise level. Hypoglycemia is best avoided by adopting a conservative approach to insulin dosing and a twice-daily dosing regimen. Good glycemic control is readily achieved in most diabetic dogs with twice-daily administration of lente, NPH, or premixed combinations of regular and NPH insulin. The diet fed should primarily be palatable and nutritionally balanced. Good clinical control is readily achieved with adult dog maintenance diets. Improved glycemic control may be achieved in some diabetic dogs if the diet contains increased insoluble fiber. Polydipsia, weight loss, lethargy, persistent negative glucosuria, or clinical signs of hypoglycemia indicate that the diabetic dog may require adjustment of the insulin dose. Evaluation of a serial blood glucose curve is required to determine how to make the dosage adjustment.
Influence of a high fibre diet on glycaemic control and quality of life in dogs with diabetes mellitus. P. A. Graham, I. E. Maskell, J. M. Rawlings, A. S. Nash, P. J. Markwell. J. Small Anim. Prat. February 2002;43(2):67-73. Quote: A study was undertaken to evaluate a high fibre diet used in the management of 10 dogs with naturally occurring insulin-dependent diabetes mellitus. Following baseline measurements of health and glycaemic control, the dogs were fed a canned diet containing a blend of insoluble and soluble dietary fibres and were monitored during the ensuing four months. Switching to the high fibre diet was associated with significantly lower mean 24-hour and postprandial plasma glucose concentrations, which were maintained over the study period. The high fibre diet was also associated with significant reductions in plasma concentrations of fructosamine, glycated haemoglobin, free glycerol and cholesterol, and there were significant improvements in dog activity and demeanour. Bodyweight declined during the fourth month of feeding the diet, which is likely to have resulted from underfeeding relative to increased activity. The results indicate that a high fibre diet can significantly improve glycaemic control and quality of life in dogs with diabetes mellitus.
Cavalier King Charles Spaniels: An Owner's Companion. John Evans. Crowood Press, 2003, pp. 36, 37.
Guide to Congenital and Heritable Disorders in Dogs. Dodds WJ, Hall S, Inks K, A.V.A.R., Jan 2004, Section II(85).
Study of 253 dogs in the United Kingdom with diabetes mellitus. L. J. Davison, M. E. Herrtage, B. Catchpole. Vet. Rec. 2005;156:467-471. Quote: "Clinical information and blood samples were collected from 253 dogs with naturally occurring diabetes mellitus [including 10 cavalier King Charles spaniels]. Over half of them were labrador retrievers, collies, Yorkshire terriers or crossbred dogs, and approximately 80 per cent of them were diagnosed between the ages of five and 12 years. ... The database of insured dogs suggests that the relatively high proportion of some breeds in the diabetic population, for example, labrador retrievers, might simply be due to their popularity, whereas other breeds, such as the cavalier King Charles spaniel, Tibetan terrier, samoyed and cairn terrier, seem to be over-represented in the diabetic population compared with the general population. ...The majority of the dogs were receiving insulin therapy once a day, but in the dogs receiving insulin injections twice a day there was a trend for lower serum fructosamine concentrations, suggesting better glycaemic control. The proportion of female dogs with diabetes was lower than in previous surveys. The disease was diagnosed more commonly in the winter months, a seasonal pattern also observed in human beings with diabetes, suggesting that similar environmental factors might be involved in the disease."
Analysis of Candidate Susceptibility Genes in Canine Diabetes. Andrea D. Short, Brian Catchpole, Lorna J. Kennedy, Annette Barnes, Neale Fretwell, Chris Jones, Wendy Thomson, and William E. R. Ollier. J. Heredity; 2007:98(5):518–525. Quote: "This study demonstrates that a number of the candidate genes previously associated with human T1D also appear to be associated with canine diabetes and identifies an IL-10 haplotype which is associated with diabetes in the Cavalier King Charles Spaniel. This suggests that canine diabetes is an excellent comparative and spontaneously occurring disease model of human T1D. ... Of the high-risk alleles, the most striking observation was the number of IL-10 alleles showing association to diabetes in the Cavalier King Charles Spaniel. This breed showed 8 positive associations for this gene, all of which had OR between 3.30 and 4.05. Such a high number of associated alleles in this gene were indicative of a risk haplotype for this breed. IL-10 did not show any associations for any other breed in this analysis. ... In the Cavalier King Charles Spaniel, IL-10 showed a high number of SNPs* to be associated with DM. This is supportive that the gene contributes to disease susceptibility/resistance as the associations are found with more than one SNP. Given the high level of LD that exists across the canine genome, these SNPs are likely to be in strong allelic association and represent haplotypes; such heterogeneity would therefore be better assessed through haplotype analysis. Haplotype analysis in this study identified an associated haplotype in the Cavalier King Charles Spaniel with a v2 of 7.48 (P 5 0.005). This haplotype did not appear to be significant in the other breeds ... " *SNP stands for a "single-nucleotide polymorphism", which is a DNA sequence variation occurring when a single nucleotide in the genome (or other shared sequence) differs between members of a species or between paired chromosomes in an individual.
Evidence of cardiac autonomic neuropathy in dogs with diabetes mellitus. S. Kenefick, N. Parker, L. Slater, A. Boswood. Vet Rec. 2007;161:83-88. Quote: "The NeuroScope, a specific and sensitive indicator of cardiac vagal tone, was used to look for differences in autonomic tone between 25 dogs with naturally occurring diabetes mellitus [including 2 cavalier King Charles spaniels] and 23 healthy control dogs, to determine whether there was any correlation between the dogs' cardiac vagal tone, the duration of diabetes and the adequacy of glycaemic control. The cardiac index of parasympathetic activity (cipa) was determined for each dog over a period of 2600 heartbeats. The mean, median and modal cipa values were significantly lower in the diabetic dogs than in the healthy dogs. There was no significant relationship between the cipa values and the duration of disease or the adequacy of recent glycaemic control in the diabetic dogs, but there was a significant inverse relationship between the cipa values and the bodyweight of the diabetic dogs that was not evident in the normal dogs. The conclusions were based on a 500-heartbeat interval selected from the 2600 heartbeats recorded."
T cell cytokine gene polymorphisms in canine diabetes mellitus. Short AD, Catchpole B, Kennedy LJ, Barnes A, Lee AC, Jones CA, Fretwell N, Ollier WE. Vet Immunol Immunopathol. 2009 Mar 15;128(1-3):137-46. Quote: "Insulin-deficiency diabetes in dogs shares some similarities with human latent autoimmune diabetes of adults (LADA). Canine diabetes is likely to have a complex pathogenesis with multiple genes contributing to overall susceptibility and/or disease progression. An association has previously been shown between canine diabetes and MHC class II genes, although other genes are also likely to contribute to the genetic risk. Potential diabetes susceptibility genes include immuno-regulatory TH1/TH2 cytokines such as IFNgamma, IL-12, IL-4 and IL-10. We screened these candidate genes for single nucleotide polymorphisms (SNPs) in a range of different dog breeds using dHPLC analysis and DNA sequencing. Thirty-eight of the SNPs were genotyped in crossbreed dogs and seven other breed groups (Labrador Retriever, West Highland White Terrier, Collie, Schnauzer, Cairn Terrier, Samoyed and Cavalier King Charles Spaniel), which demonstrated substantial intra-breed differences in allele frequencies. When SNPs were examined for an association with diabetes by case:control analysis significant associations were observed for IL-4 in three breeds, the Collie, Cairn Terrier and Schnauzer and for IL-10 in the Cavalier King Charles Spaniel. These results suggest that canine cytokine genes regulating the TH1/TH2 immune balance might play a contributory role in determining susceptibility to diabetes in some breeds."
Lack of advantage of high-fibre, moderate-carbohydrate diets in dogs with stabilised diabetes. L. M. Fleeman, J. S. Rand, P. J. Markwell. J. Small Anim. Pract. October 2009;50(11):604-614. Quote: Objectives:To assess the influence of high-fibre, moderate-carbohydrate diets with two levels of dietary fat, compared with a commercial diet with moderate-fibre, low-carbohydrate and higher fat, on insulin requirement, glycaemic control and lipid profile of dogs with stabilised diabetes. Methods: Twelve dogs with spontaneous diabetes mellitus were studied. Glycaemic control was evaluated by plasma fructosamine, glycosylated haemoglobin and 48-hour serial blood glucose measurements. The insulin dosage required to maintain clinical stability was also determined. Lipid profiles comprised serial measurements of plasma cholesterol, triglyceride, free glycerol and free fatty acids. Data were analysed using analysis of variance. Results:There were no significant differences in insulin requirement or glycaemic control among diets. Weight loss occurred when the dogs were fed the high-fibre, moderate-carbohydrate, moderate-fat diet (P<0·002), whereas weight was maintained with the other two diets. The high-fibre, moderate-carbohydrate, moderate-fat diet resulted in lower mean plasma cholesterol compared with either of the higher-fat diets (P≤0·003), and lower mean plasma triglyceride (P=0·060), free fatty acid (P<0·001) and free glycerol (P=0·015) than the commercial diet. Clinical Significance:For stable diabetic dogs, high-fibre, moderate-carbohydrate diets offered no significant advantage compared with a commercial diet with moderate fibre and low carbohydrate. Diets with high fibre, moderate carbohydrate and moderate fat should not be routinely recommended for dogs with thin body condition.
Topical KINOSTAT ameliorates the clinical development and progression of cataracts in dogs with diabetes mellitus. Peter F. Kador, Terah R. Webb, Dineli Bras, Kerry Ketring, and Milton Wyman. Vet. Ophth. Nov.2010;13(6):363–368. Quote: "Objective: To determine whether topical administration of the aldose reductase inhibitor Kinostat can ameliorate the onset or progression of cataracts in dogs with naturally occurring diabetes mellitus (DM). Materials and Methods: A randomized, prospective, double-masked placebo control pilot study was conducted with 40 dogs newly diagnosed with DM with no or minimal lens changes. Twenty-eight dogs received Kinostat and 12 dogs received placebo. Procedures: Owners administered the agent into both eyes three times daily for 1 year and compliance was monitored with log sheets. Cataract severity was assessed on a scale of 0–3. Results After 12 months of treatment, the cataract score in the placebo group significantly increased with seven dogs (14 eyes) developing mature cataracts, two dogs (4 eyes) developing cortical opacities, and one dog (2 eyes) developing equatorial vacuoles with mild punctate cortical opacities. In contrast, the cataract score in the KinostatTM treated dogs was significantly less with seven developing anterior equatorial vacuoles, two developing incipient anterior cortical cataracts, and four developing mature cataracts. In fact, the cataract scores of the Kinostat group at 12 months did not significantly increase from the score at the time of enrollment. The HbA1C values between the two groups after 12 months of treatment were similar, and no blood levels of Kinostat were found in any enrolled dog. Conclusion: The onset and/or progression of cataracts in dogs with DM can be significantly delayed by topical administration of Kinostat."
Diabetes mellitus. Canine Inherited Disorders Database. 2011.
Genetic Connection: A Guide to Health Problems in Purebred Dogs, Second Edition. Lowell Ackerman. July 2011; AAHA Press; pg 66. Quote: "An interleukin-10 haplotype appears to be associated with diabetes mellitus in the Cavalier King Charles spaniel."
A diet lower in digestible carbohydrate results in lower postprandial glucose concentrations compared with a traditional canine diabetes diet and an adult maintenance diet in healthy dogs. K.F. Elliott, J.S. Rand, L.M. Fleeman, J.M. Morton, A.L. Litster, V.C. Biourge, P.J. Markwell. Research in Vet. Sci. August 2012;93(1):288-295. Quote: The aim of this study was to compare the effects of three diets with varying macronutrient and fibre contents on postprandial plasma glucose, triglyceride, free fatty acid, and insulin concentrations over a 12 h period in 12 healthy neutered lean dogs. Each diet was fed to each dog for 3 weeks in a three-period cross-over study. Plasma analyte concentrations were measured prior to and after a meal at the end of the third week of each period. Postprandial glucose concentrations for the moderate carbohydrate and fibre diet were 0.4–0.7 mmol/L (8–12 mg/dL) lower than for both higher carbohydrate diets (p ⩽ 0.02). Postprandial glucose, insulin, and triglyceride concentrations in some dogs did not return to baseline by 12 h after feeding of each of the three diets. These results indicate that the moderate carbohydrate and fibre diet warrants evaluation in diabetic dogs. Variables should be measured over at least 12 h after feeding to fully evaluate postprandial dietary effects on these analytes.
Genetics of canine diabetes mellitus: Are the diabetes susceptibility genes identified in humans involved in breed susceptibility to diabetes mellitus in dogs? Brian Catchpole, Jamie P. Adams, Angela L. Holder, Andrea D. Short, William E.R. Ollier, Lorna J. Kennedy. Vet.J. Dec. 2012. Quote: "Diabetes mellitus is a common endocrinopathy in companion animals, characterised by hyperglycaemia, glycosuria and weight loss, resulting from an absolute or relative deficiency in the pancreatic hormone insulin. There are breed differences in susceptibility to diabetes mellitus in dogs, with the Samoyed breed being overrepresented, while Boxers are relatively absent in the UK population of diabetic dogs, suggesting that genetic factors play an important role in determining susceptibility to the disease. A number of genes, linked with susceptibility to diabetes mellitus in humans, are associated with an increased risk of diabetes mellitus in dogs, some of which appear to be relatively breed-specific. Diabetes mellitus in dogs has been associated with major histocompatibility complex (MHC) class II genes (dog leucocyte antigen; DLA), with similar haplotypes and genotypes being identified in the most susceptible breeds. A region containing a variable number of tandem repeats (VNTR) and several polymorphisms have been identified in the canine insulin gene, with some alleles associated with susceptibility or resistance to diabetes mellitus in a breed-specific manner. Polymorphisms in the canine CTLA4 promoter and in other immune response genes are associated with susceptibility to diabetes mellitus in a number of pedigree breeds. Genome wide association studies are currently underway that should shed further light on the genetic factors responsible for the breed profile seen in the diabetic dog population."
Treatment of Diabetes and Long-term Survival Following Insulin and Glucokinase Gene Therapy. David Callejas, Christopher John Mann, Eduard Ayuso, Ricardo Lage, Iris Grifoll, Carles Roca, Anna Andaluz, Rafael Ruiz-de Gopegui, Joel Montane, Sergio Muńoz, Tura Ferre, Virginia Haurigot, Shangzhen Zhou, Jesus Ruberte, Federico Mingozzi, Katherine High, Felix Garcia, and Fatima Bosch. Diabetes. Feb. 2013. Quote: "Diabetes is associated with severe secondary complications, caused largely by poor glycemic control. Treatment with exogenous insulin fails to prevent these complications completely, leading to significant morbidity and mortality. We previously demonstrated that it is possible to generate a “glucose sensor” in skeletal muscle through co-expression of glucokinase (Gck) and insulin (Ins), increasing glucose uptake and correcting hyperglycemia in diabetic mice. Here, we demonstrate long-term efficacy of this approach in a large animal model of diabetes. A one-time intramuscular administration of adenoassociated viral vectors of serotype 1 (AAV1) encoding for Gck and Ins in diabetic dogs resulted in normalization of fasting glycemia, accelerated disposal of glucose after oral challenge, and no episodes of hypoglycemia during exercise for >4 years after gene transfer. This was associated with recovery of body weight, reduced glycosylated plasma proteins levels, and long-term survival without secondary complications. Conversely, exogenous insulin or gene transfer for Ins or Gck alone failed to achieve complete correction of diabetes, indicating that the synergistic action of Ins and Gck are needed for full therapeutic effect. This study provides the first proof-of-concept in a large animal model for a gene transfer approach to treat diabetes."
Glargine insulin for treatment of naturally occurring diabetes mellitus in dogs. Rebecka S. Hess, Kenneth J. Drobatz. JAVMA Oct. 2013;243(8):1154-1161. Quote: "Objective: To evaluate the effects of twice-daily glargine insulin administration in dogs with diabetes mellitus. Design: Open-label, prospective clinical trial. Animals: 10 dogs with naturally occurring diabetes mellitus. Procedures: Dogs with poorly regulated or newly diagnosed diabetes mellitus were enrolled if their owners agreed to return them to the hospital at 1- to 3-week intervals for 4 follow-up visits. During each follow-up visit, blood glucose concentrations were measured every 2 hours for at least 10 hours after feeding a diet high in insoluble fiber and after administration of glargine insulin (time 0). The initial glargine insulin dosage was 0.5 U/kg (0.23 U/lb) SC twice daily. Results: All dogs had well-regulated diabetes mellitus at a mean ± SD of 38 ± 14 days (median, 43 days; range, 7 to 55 days) following study enrollment. At the time diabetes mellitus was well regulated, mean glargine insulin dosage was 0.5 ± 0.15 U/kg (0.23 ± 0.068 U/lb; median, 0.5 U/kg; range, 0.32 to 0.67 U/kg [0.15 to 0.30 U/lb]) twice daily, and 3 dogs were receiving a dosage < 0.4 U/kg (0.18 U/lb). In dogs with well-regulated diabetes mellitus, the mean minimum blood glucose concentration (163 ± 89 mg/dL; 95% confidence interval, 100 to 227 mg/dL) was detected 2 hours after administration of glargine insulin and the mean maximum blood glucose concentration (230 ± 95 mg/dL; 95% confidence interval, 64 to 323 mg/dL) was detected 12 hours after administration of glargine insulin. There was no significant difference between mean minimum and mean maximum blood glucose concentrations nor were there significant differences between blood glucose concentrations measured at other time points. Blood glucose concentration < 80 mg/dL was measured at least once in 7 of 10 dogs. Conclusions and Clinical Relevance: Results of the present study suggested that, in diabetic dogs fed a diet high in insoluble fiber, glargine insulin is a peakless insulin that does not induce a distinct blood glucose concentration nadir. For glargine insulin, 0.3 U/kg (0.136 U/lb) SC twice daily is recommended as an initial dosage."
A case–control study between interleukin-10 gene variants and periodontal disease in dogs. Carlos Albuquerque, Francisco Morinha, João Requicha, Isabel Dias, Henrique Guedes-Pinto, Carlos Viegas, Estela Bastos. Gene. April 2014;539(1):75-81. Quote: "Periodontal disease (PD) refers to a group of inflammatory diseases caused by bacterial plaque in the periodontium and ranges from an early stage (gingivitis) to an advanced stage (periodontitis). It is a multifactorial disease that results from the interaction of the host defence mechanisms with the plaque microorganisms. PD has an enormous impact on human medicine and veterinary medicine due to its high prevalence as well as its local and systemic implications. Dog model has been extensively used in oral disease research, contributing significantly to the current understanding of periodontology. The most important clinical aspects of canine PD were considered in this work and the various animal models were examined with emphasis on the role of the dog as the most useful model for understanding human PD and to develop new therapeutic and preventive measures. In recent decades, it has been consolidated the idea of the genetics influence in PD by controlling the inflammatory process severity and the therapy responses. Various single nucleotide polymorphisms have been identified as risk factors, mainly in genes responsible for molecules involved in immunoregulation and/or metabolism, but many questions still remain. In canine PD, this is a completely unexplored issue but a highly relevant and promising research field namely because the strong similarity between canine and human disease provides the possibility to share the knowledge attained from one species to another, with mutual benefits. Following a comparative genomics approach to identify the most promising candidate genes, the main goal of this work was to contribute for a better characterization of canine PD, particularly in terms of genetic basis. Five candidate genes (IL1A, IL1B, IL10, IL6 and LTF) encoding molecules with recognized relevant role in the PD pathogenesis (interleukin-1α, interleukin-1β, interleukin-10, interleukin-6 and lactotransferrin, respectively) were selected and case-control studies were delineated, in which a molecular analysis of each gene was performed to identify genetic variations and to evaluate its possible association with PD. It was hypothesized that in canine PD, similar to human PD, a disfunction or a dysregulated production of these molecules resulting from genetic variations can be part of the explanation for the differences in disease susceptibility between individuals. A total of twenty-six genetic variations were identified and analyzed, eight in the IL1A and IL1B genes, seven in the IL10 gene, three in the IL6 gene, and eight in the LTF gene. The IL1A/1_g.388A>C and IL1A/1_g.521T>A variations showed statistically significant differences between groups [adjusted OR (95%CI): 0.15 (0.03-0.76), p=0.022; 5.76 (1.03-32.1), p=0.046, respectively], meaning that, in the studied population, the IL1A/1_g.388C allele is associated with a decreased PD risk, whereas the IL1A/1_g.521A allele is associated with an increased risk. Regarding all the others variations, no statistically significant differences were detected, but the IL1A/2_g.515G>T, IL10/2_g.285G>A, IL6/2_g.105G>A, LTF/3_g.411C>T, LTF/3_g.420G>A and LTF/3_g.482G>A variations resulted in a change of encoded amino acid, which may alter protein structure and function, as demonstrated by different bioinformatics tools. Before the molecular analysis of the IL10 gene, two additional studies were delineated. Considering that no clear consensus has been reached about the association of IL10 polymorphisms and human PD, a meta-analysis of all available studies was performed. It was found statistically significant association of IL10-819(-824)C>T and IL10-592(-597)C>A polymorphisms, with IL10-819(-824)T and -592(-597)A alleles conferring a relative increased risk for chronic periodontits in Caucasians. Additionally, a study to evaluate the levels of interleukin-10 in plasma of dogs with periodontitis was delineated assessing a possible correlation between these levels and periodontal condition, being found lower levels in the periodontitis group comparing with the control group. The outcome from this work suggests that dog IL1A, IL1B, IL10, IL6 and LTF genes, as occurs in the human orthologous genes, are highly polymorphic with genetic variants that may be important in PD susceptibility. ... Short et al. (2007) developed an analysis of candidate susceptibility genes in canine diabetes, including IL10 gene, selected taking in account the previous associations described in human diabetes. These authors found various polymorphisms in dog IL10 gene associated with diabetes mellitus in the Cavalier King Charles Spaniel. ... The results obtained for the IL1A are particularly relevant, but this is the first work in this issue and further studies are essential to reinforce these findings and to clarify its biological importance; as well as other studies with different candidate genes. But, it is undeniable that advances in this area are fundamental to understand properly the complex causal pathways of PD and to improve the clinical management of PD, particularly with the development of novel strategies of risk assessment. A candidate gene approach supported in comparative genomics tools is a promising path of research to achieve these objectives, which may lead to great benefits in human and veterinary periodontology, adding important knowledge to design new preventive and therapeutic strategies, and ultimately to improve health in both humans and dogs.
Searching for “monogenic diabetes” in dogs using a candidate gene approach. Andrea D. Short, Angela Holder, Simon Rothwell, Jonathan Massey, Rachel Scholey, Lorna J. Kennedy, Brian Catchpole, William E. R. Ollier. Canine Genetics & Epidemiology. July 2014. Quote: "Background: Canine diabetes is a common endocrine disorder with an estimated breed-related prevalence ranging from 0.005% to 1.5% in pet dogs. Increased prevalence in some breeds suggests that diabetes in dogs is influenced by genetic factors and similarities between canine and human diabetes phenotypes suggest that the same genes might be associated with disease susceptibility in both species. Between 1-5% of human diabetes cases result from mutations in a single gene, including maturity onset diabetes of the adult (MODY) and neonatal diabetes mellitus (NDM). It is not clear whether monogenic forms of diabetes exist within some dog breeds. Identification of forms of canine monogenic diabetes could help to resolve the heterogeneity of the condition and lead to development of breed-specific genetic tests for diabetes susceptibility. Results: Seventeen dog breeds [including 107 cavalier King Charles spaniels] were screened for single nucleotide polymorphisms (SNPs) in eighteen genes that have been associated with human MODY/NDM. Six SNP associations were found from five genes, with one gene (ZFP57) being associated in two different breeds. Conclusions: Some of the genes that have been associated with susceptibility to MODY and NDM in humans appear to also be associated with canine diabetes, although the limited number of associations identified in this study indicates canine diabetes is a heterogeneous condition and is most likely to be a polygenic trait in most dog breeds."
Diabetes mellitus and pancreatitis – cause or effect? Lucy J. Davison. J. Small Animal Practice. January 2015;56(1):50-59. Quote: "Diabetes mellitus and pancreatitis are two distinct diseases encountered commonly in small animal practice. Whilst the clinical signs of diabetes mellitus are usually unmistakeable, a firm diagnosis of pancreatitis can prove more elusive, as clinical signs are often variable. ... Recent work to examine the breed-related prevalence of CP in postmortem pancreata from first opinion practice in the UK suggested that histological evidence of CP in dogs is present in approximately 34% of cadavers, with Cavalier King Charles spaniels (CKCS), collies and boxers being over-represented (Watson et al. 2007). ... Over the past 10 to 15 years, despite the fact that the clinical signs of diabetes mellitus are remarkably consistent, it has become more apparent that the underlying pathology of diabetes mellitus in dogs and cats is heterogeneous, with exocrine pancreatic inflammation accompanying diabetes mellitus in a number of cases. However, the question remains as to whether the diabetes mellitus causes the pancreatitis or whether, conversely, the pancreatitis leads to diabetes mellitus – as there is evidence to support both scenarios. The concurrence of diabetes mellitus and pancreatitis has clinical implications for case management as such cases may follow a more difficult clinical course, with their glycaemic control being “brittle” as a result of variation in the degree of pancreatic inflammation. Problems may also arise if abdominal pain or vomiting lead to anorexia. In addition, diabetic cases with pancreatitis are at risk of developing exocrine pancreatic insufficiency in the following months to years, which can complicate their management further."
Extreme Beta-Cell Deficiency in Pancreata of Dogs with Canine Diabetes. Emily J. Shields, Carol J. Lam, Aaron R. Cox, Matthew M. Rankin, Thomas J. Van Winkle, Rebecka S. Hess, Jake A. Kushner. Plos One. June 2015. Quote "The pathophysiology of canine diabetes remains poorly understood, in part due to enigmatic clinical features and the lack of detailed histopathology studies. Canine diabetes, similar to human type 1 diabetes, is frequently associated with diabetic ketoacidosis at onset or after insulin omission. However, notable differences exist. Whereas human type 1 diabetes often occurs in children, canine diabetes is typically described in middle age to elderly dogs. Many competing theories have been proposed regarding the underlying cause of canine diabetes, from pancreatic atrophy to chronic pancreatitis to autoimmune mediated β-cell destruction. It remains unclear to what extent β-cell loss contributes to canine diabetes, as precise quantifications of islet morphometry have not been performed. We used high-throughput microscopy and automated image processing to characterize islet histology in a large collection of pancreata of diabetic dogs. Diabetic pancreata displayed a profound reduction in β-cells and islet endocrine cells. Unlike humans, canine non-diabetic islets are largely comprised of β-cells. Very few β-cells remained in islets of diabetic dogs, even in pancreata from new onset cases. Similarly, total islet endocrine cell number was sharply reduced in diabetic dogs. No compensatory proliferation or lymphocyte infiltration was detected. The majority of pancreata had no evidence of pancreatitis. Thus, canine diabetes is associated with extreme β-cell deficiency in both new and longstanding disease. The β-cell predominant composition of canine islets and the near-total absence of β-cells in new onset elderly diabetic dogs strongly implies that similar to human type 1 diabetes, β-cell loss underlies the pathophysiology of canine diabetes."
Canine diabetes mellitus: what is new? Stijn Niessen. Companion Animal. August 2015;20(8):443-446. Quote: Recent developments in genetic research have increased our understanding of the most common form of diabetes affecting pet dogs. This research starts to explain why certain breeds seem more susceptible to developing diabetes; in the long run it could lead to revolutionary screening and prevention strategies. Nevertheless, the genetics do not completely explain the ultimate establishment of the diabetic state, therefore the search continues for other (epigenetic and environmental) factors involved in this process. Clinicians should remain open-minded for the existence of a variety of different types of canine diabetes mellitus. Knowing the aetiology is important, since this will enable development of superior treatment strategies. An entire female diabetic dog usually benefits from prompt neutering, since diabetic remission is possible in a subset of cases and achieving diabetic control will prove easier even if remission is not achieved. Currently, routine treatment of diabetic dogs is based on matching insulin activity, usually provided by twice-daily insulin injections, with post-prandial hyperglycaemia. Being strategic in the timing of injection versus feeding can prove beneficial. Studies with relatively novel drugs such as GLP-1 agonists are currently ongoing and seem promising. In the future, canine diabetes mellitus will probably best be treated through complete pancreatic beta-cell replacement, either by transplantation, regenerative medicine or the use of an artificial pancreas. Gene therapy could play a distinct role and has already been implemented in small trials. Development of a veterinary practice-based diabetes team working closely with dog owners will enable individualisation and optimisation of diabetic pet care whichever shape it takes in the future. Veterinary nurses should play a crucial role in such a team, mirroring the situation in human diabetic care. Where possible, clinic-wide diabetes protocols should be adapted and made flexible, paying particular attention to co-morbidities and their impact on glycaemic control, as well as on the quality of life of the diabetic dog and owner. The use of available quality of life measurement tools should be actively encouraged by any clinic taking individualised diabetic care seriously.
What's in a Name? Classification of Diabetes Mellitus in Veterinary Medicine and Why It Matters. Gilor C, Niessen SJ, Furrow E, DiBartola SP. J. Vet. Intern. Med. July 2016;30(4):927-940. Quote: Diabetes Mellitus (DM) is a syndrome caused by various etiologies. The clinical manifestations of DM are not indicative of the cause of the disease, but might be indicative of the stage and severity of the disease process. Accurately diagnosing and classifying diabetic dogs and cats by the underlying disease process is essential for current and future studies on early detection, prevention, and treatment of underlying disease. Here, we review the current etiology-based classification of DM and definitions of DM types in human medicine and discuss key points on the pathogenesis of each DM type and prediabetes. We then review current evidence for application of this etiology-based classification scheme in dogs and cats. In dogs, we emphasize the lack of consistent evidence for autoimmune DM (Type 1) and the possible importance of other DM types such as DM associated with exocrine pancreatic disease. While most dogs are first examined because of DM in an insulin-dependent state, early and accurate diagnosis of the underlying disease process could change the long-term outcome and allow some degree of insulin independence.
Effects of pea with barley and less-processed maize on glycaemic control in diabetic dogs. Fabio A. Teixeira, Daniela P. Machado, Juliana T. Jeremias, Mariana R. Queiroz, Cristiana F. F. Pontieri, Marcio A. Brunetto. Brit. J, Nutrition. August 2018;doi: 10.1017/S000711451800171X. Quote: The source of starch may interfere with glycaemic control in dogs, but few studies have evaluated these aspects in diabetic dogs. This study compared the effects of two isonutrient diets with different starch sources, peas and barley (PB) v. maize (Mi), on diabetic dogs. The Mi diet was processed in order to generate a lower starch gelatinisation index. In all, fifteen adult diabetic dogs without other conditions were included. The animals were fed two dry extruded rations with moderate levels of fat and starch and high levels of protein and fibre using a random, double-blind cross-over design. Glycaemic curves over 48 h were developed via continuous glucose monitoring after 60 d on each diet and with the same neutral protamine Hagedorn (NPH) insulin dosage. The following were compared: fasting, mean, maximum and minimum blood glucose, maximum and minimum glycaemia difference, glycaemic increment, area under the glycaemic curve, area under the glycaemic increment curve and serum fructosamine concentration. Paired t tests or Wilcoxon signed-rank tests were used to compare the amount of food and nutrients ingested and the dietary effects on glycaemic variables between the diets. Dogs fed the PB diet presented a lower average mean interstitial glucose (P=0·01), longer mean hypoglycaemic time (P<0·01), shorter mean hyperglycaemic time (P<0·01) and smaller difference between maximum and minimum blood glucose levels (P=0·03). Thus, the processing applied to the Mi diet was not sufficient to achieve the same effects of PB on glycaemic control in diabetic dogs.
Changes of cardiac function in diabetic dogs. Vichit, P., Rungsipipat, A., & Surachetpong, S. D. J. Vet. Cardiol. September 2018; doi: 10.1016/j.jvc.2018.08.001. Quote: Objective: This study aimed to evaluate cardiac function and compare the concentration of cardiac biomarkers including cardiac troponin I (cTnI), galectin-3 (Gal-3), and N-terminal pro B-type natriuretic peptides (NT-proBNP) in diabetic and control dogs. Animals: Thirty-nine dogs were included. The diabetic and control groups consisted of 19 and 20 dogs, respectively. Methods: Plasma cTnI, Gal-3, and NT-proBNP concentrations were measured in the diabetic and control groups. Echocardiography was performed in all dogs to evaluate cardiac structure and function. Echocardiographic values and cardiac biomarker concentrations between the two groups were compared with the Mann-Whitney U test. The p-value < 0.05 was considered statistical significance. Results: No evidence of cardiac structural changes was detected in diabetic dogs on two-dimensional echocardiography. The echocardiographic values of diabetic and control dogs were within reference intervals. Echocardiographic changes indicating diastolic dysfunction assessed by spectral flow Doppler echocardiography and tissue Doppler imaging were found in diabetic dogs (42.10%) compared with control dogs (10.00%; p = 0.022). Diabetic dogs with durations of diabetes mellitus > 1 year had an increased left ventricular wall thickness and echocardiographic changes suggesting diastolic dysfunction compared with those with duration of diabetes mellitus < 1 year. No evidence of systolic dysfunction was detected in diabetic dogs. No significant difference in plasma cTnI, Gal-3, and NT-proBNP concentrations was found between the two groups. Conclusions: Echocardiographic changes suggested that left ventricular diastolic dysfunction was detected in diabetic dogs without changes in the concentration of cardiac biomarkers including cTnI, Gal-3, and NT-proBNP compared with the age- and breed-matched control dogs.
An Epidemiological Study of Dogs with Diabetes Mellitus Attending Primary Care Veterinary Clinics in Australia. Linda M. Fleeman, Samuel Yoon, Bethany Wilson, Caroline Mansfield, Paul McGreevy. J. Vet. Intern. Med. September 2019;doi: 10.1111/jvim.15597;#EN08:59. Quote: The objective was to determine the prevalence, risk factors, and comorbidities associated with diabetes mellitus (DM) in dogs attending primary care veterinary clinics in Australia. Electronic medical records of dogs (n = 134,329) that attended 152 clinics during 2017 were sourced through VetCompass Australia. A nested case-control study was used, with cases and age-matched controls from the same cohort and time-frame frequency matched at a 1:4 ratio. Logistic regression models analyzed risk factors, and binary logistic regression analyses and Fischer’s exact tests were performed. Four hundred and eighty-one dogs with DM were identified resulting in a prevalence of 0.36%. Breeds (and their crosses) with significantly higher odds of having DM were Australian terriers (Odds Ratio (OR) 7.93), Siberian huskies (OR 6.24), English springer spaniels (OR 5.37), West highland white terriers (OR 4.85), Miniature schnauzers (OR 3.47), all types of Poodles (OR 3.41), Bichón Frisés (OR 3.41), Schnauzers (OR 3.18), and Cavalier King Charles spaniels (OR 1.84). German shepherds (OR 0.11), Golden retrievers (OR 0.09), and Boxers (OR 0.00) had a lower risk of DM. Labradoodles were diagnosed more often than purebred Labradors (P = 0.04) and did not differ from Poodles (P = 0.81). Desexed male dogs had a higher risk of DM compared with entire males (P = 0.02) and desexed females (P = 0.02). Comorbidities associated with canine DM included suspected pancreatitis (OR 10.58), cataracts (OR 9.80), hyperadrenocorticism (OR 6.21), urinary tract infection (OR 5.09), and hypothyroidism (OR 4.10). These findings will inform and guide future research on genetic and environmental influences of DM in dogs. (See the published 2020 full report of this study.)
Epidemiological study of dogs with diabetes mellitus attending primary care veterinary clinics in Australia. Samuel Yoon, Linda M Fleeman, Bethany J Wilson, Caroline S Mansfield, Paul McGreevy. Vet. Rec. February 2020; doi: 10.1136/vr.105467. Quote: Background: The objectives of this study were to establish the prevalence, risk factors and comorbidities/sequelae for diabetes mellitus (DM) in Australian dogs presented to first-opinion veterinary practices. Methods: Electronic patient records of dogs (n=134,329) attending 152 veterinary clinics during 2017 were sourced through VetCompass Australia. They included 418 dogs with DM; a prevalence of 0.36 per cent in Australian dogs attending these veterinary clinics. By comparing with the reference group of rarer breeds and unidentified crossbreeds, multivariable modelling was used to reveal breeds (and their crosses) with significantly higher odds of having DM. Results: The results revealed that breeds (and their crosses) with significantly higher odds of having DM were Australian terriers, Siberian huskies), English springer spaniels, West Highland white terriers, miniature schnauzers, all types of poodles, bichon frises, schnauzers and cavalier King Charles spaniels (CKCS). Breeds with lower risk were German shepherd dogs, golden retrievers and boxers (no cases identified). Fisher’s exact tests showed that labradoodles were diagnosed significantly more often than purebred Labradors and did not differ significantly from poodles. Cavoodles did not differ significantly from either CKCS or poodles. Spoodles were significantly less diagnosed than poodles but did not differ from cocker spaniels. Desexed male dogs had a higher odds of DM than entire male and desexed female dogs. Comorbidities / sequelae associated with canine DM included suspected pancreatitis, cataracts, hyperadrenocorticism, urinary tract infection and hypothyroidism. Conclusions: Breeds at most risk included Australian terriers and Siberian huskies as previously reported, as well as, for the first time, English springer spaniels. In contrast to other populations where there is female predisposition for DM, desexed male dogs in Australia were at increased risk for DM compared with both entire males and desexed females. This predisposition for desexed males to develop DM warrants further investigation. (See also the 2019 ACVIM Forum abstract of this study.)
Diabetes mellitus in dogs attending UK primary-care practices: frequency, risk factors and survival. Angela M. Heeley, Dan G. O’Neill, Lucy J. Davison, David B. Church, Ellie K. Corless, Dave C. Brodbelt. Canine Med. & Genetics. June 2020; doi: 10.1186/s40575-020-00087-7. Quote: Background: Diabetes mellitus (DM) is an important endocrine disorder of dogs. The objectives of this study were to estimate prevalence and incidence of DM in dogs, and to explore risk factors for DM and the survival of DM cases in primary-care clinics in the UK. Results: A case-control study nested in the cohort of dogs (n = 480,469) aged ≥3 years presenting at 430 VetCompass clinics was used to identify risk factors for DM, using multivariable logistic regression. Overall 409 new and 863 pre-existing DM cases (total 1272) were identified in 2016, giving an apparent annual prevalence of 0.26%, and an annual incidence risk of 0.09% in dogs aged ≥3 years. Factors associated with increased odds for DM diagnosis were all age categories > 8 years, female entire dogs and male neutered dogs compared to male entire dogs, Border Terriers and West Highland White Terriers (WHWT) compared to crossbreeds. Dogs that had received previous glucocorticoid treatment and those with concurrent conditions (documented obese, pancreatitis, hyperadrenocorticism) also had increased odds for DM diagnosis. Increased hazard of death following diagnosis of DM was shown in dogs that were ≥ 10 years age, Cocker Spaniels compared to crossbreeds, had a blood glucose (BG) level at diagnosis > 40 mmol/L compared to < 20 mmol/L at diagnosis, or had received previous glucocorticoid treatment. Dogs at reduced hazard of death included neutered dogs, Border Collies, and those starting insulin treatment. Conclusions: Certain breeds and concurrent health conditions are associated with an increased risk of DM. In addition to certain signalment factors, a high BG level at diagnosis and prior glucocorticoid treatment were adversely associated with survival of dogs with DM.
Genetics of canine diabetes mellitus part 1: Phenotypes of disease. Alice L. Denyer, Brian Catchpole, Lucy J. Davison, Canine Diabetes Genetics Partnership. Vet. J. April 2021; doi: 10.1016/j.tvjl.2021.105611. Quote: This two-part article discusses the mechanisms by which genetic variation can influence the risk of complex diseases, with a focus on canine diabetes mellitus. In Part 1, presented here, the importance of accurate methods for classifying different types of diabetes will be discussed, since this underpins the selection of cases and controls for genetic studies. Conclusions: In the first of this two-part article, the classification systems for human and canine DM have been briefly discussed and the importance of precise classification in diabetes genetic studies has been outlined. Furthermore, the ways in which genetic variants may contribute to risk of a complex disease have been introduced. Highlights: • The multiple forms of human diabetes mellitus (DM) have different underlying causes. • Canine DM is likely to be heterogeneous, similar to human DM. • Identifying genetic risk factors is important to improve understanding of canine DM. • Greater understanding of canine DM aetiology will improve disease classification. • Increasing precise disease phenotyping will greatly benefit genetic studies.
Genetics of canine diabetes mellitus part 2: Current understanding and future directions. Alice L. Denyer, Canine Diabetes Genetics Partnership, Brian Catchpole, Lucy J.Davison. Vet. J. April 2021; doi: 10.1016/j.tvjl.2021.105612. Quote: Part 2 of this 2-part review, presented here, describes in more detail our current understanding of the genetics of canine diabetes mellitus compared to our knowledge of the human disease. Ongoing work to further develop our knowledge, using new technologies, are also introduced. Highlights: • Large scale genetic studies have been key in human diabetes mellitus (DM) research. • Genetic studies in canine DM have primarily used a candidate gene approach, to date. • Recent technological advances have great potential for canine DM genetic research. • The population structure of dogs is important for study of complex genetic diseases.
Assessment of glucocorticoid and antibiotic exposure as risk factors for diabetes mellitus in selected dog breeds attending UK primary-care clinics. Angela M. Heeley, Dave C. Brodbelt, Dan G. O’Neill, David B. Church, Lucy J. Davison. Vet. Rec. May 2023; doi: 10.1002/vetr.2785. Quote: Background: Diabetes mellitus (DM) is an important endocrine disorder in dogs. This study explored prior exposure to glucocorticoids or antibiotic treatment as risk factors for developing DM in dogs attending primary-care VetCompass clinics in the UK. Methods: A breed frequency matched case–control study nested in a cohort of dogs (n = 480,469) aged 3 years or over was used to explore associations between glucocorticoid and antibiotic exposure and the odds of developing DM. Results: A total of 565 cases and 2179 controls were included. ... The breeds most commonly exposed to glucocorticoids were West Highland White Terriers (20.7% [19/92] cases and 6.8% [25/370] controls), Tibetan Terriers (20.0% [3/15] cases and 3.6% [2/56] controls) and Cavalier King Charles Spaniels (13.6% [3/22] cases and 3.5% [3/85] controls). ... Dogs with DM had over four times the odds of exposure to glucocorticoids within 6 weeks prior to diagnosis (odds ratio [OR] 4.07, 95% confidence interval [CI] 2.41– 6.89, p < 0.001) compared to controls within 6 weeks prior to a randomly selected quasi-date of diagnosis. Dogs that had only one unique documented antibiotic course had a decreased odds of developing DM (OR 0.65, 95% CI 0.46–0.91, p = 0.012) compared to dogs that had no documented courses of antibiotics. Limitations: This study only included selected breeds, so the results may not be generalisable to all dog breeds. Conclusions: Exposure to glucocorticoids is associated with a substantial increase in the risk of developing DM for the dog breeds included in this analysis.
Epidemiology and clinical management of 1072 dogs with diabetes mellitus in a UK diabetes register. A. L. Denyer, D. G. O’Neill, D. C. Brodbelt, A. Holder, B. Catchpole, L. J. Davison. Companion Anim. Health Genet. October 2025; doi: 10.1186/s40575-025-00146-x. Quote: Background: The UK Canine Diabetes Register and Archive (UKCDRA), established in 1999, is a valuable resource of clinical data and blood samples from diabetic dogs. This study aimed to provide updated information about the epidemiology and clinical management of canine diabetes mellitus (DM) in UK veterinary practices. Method: Data from samples submitted to UKCDRA between December 2005 and December 2019 were divided into three groups according to DM aetiology: juvenile-onset, adult-onset non-dioestrus and adult-onset female entire. Epidemiological and clinical factors were analysed across groups. Breeds with more than 5 UKCDRA DM cases and/or ≥ 5000 dogs in the non-diabetic VetCompass denominator population were compared to those in the wider UK VetCompass™ population to explore breed DM risk. Results: Ninety-nine breeds were represented in the study, with 25 breeds having 5 or more diabetic cases [including 27 cavalier King Charles spaniels, which ranked 9th in number of cases]. Samoyeds and Tibetan terriers demonstrated the highest odds of DM in both of the adult-onset DM groups. Age of adult-onset DM onset co-varied with breed, with the Standard Doberman Pinscher and Rottweiler demonstrating youngest onset age. ... As a follow-up to one-way ANOVA, Dunnett’s test identified a significant difference between the mean age of diagnosis of DM in Crossbreeds (reference population) and that of the Standard Doberman Pinscher, Rottweiler, Cavalier King Charles Spaniel, Dachshund and Labrador Retriever breeds, which were all younger at the time of diagnosis than Crossbreeds (Table 2). ... Twice daily Caninsulin (40 iu/ml porcine insulin) was the most commonly reported treatment. Twice-daily rather than once-daily insulin therapy in canine DM has become more prevalent since the archive was founded. Limitations: UKCDRA does not capture detailed information on concurrent diseases or DM environmental risk factors. Conclusion: This study provides an update to an earlier UKCDRA report and demonstrates shared breed-associated risk factors for adult-onset non-dioestrus and female entire DM.


CONNECT WITH US